Emerging Markets
Biotech Sleeper (NWBO) Northwest Biotherapeutics Heats Up as Imminent DCVax-L Phase III Top Line Data Looms Large
Published
3 years agoon
By
Boe Rimes
Northwest Biotherapeutics, Inc (OTCMKTS: NWBO) has moved back into full beast mode and has been running higher in recent trading on heavy accumulation. NWBO is the darling of small caps and has proven itself as among the most resilient stocks in small caps with a huge following of investors. With a 52-week high of $2.51 and a newly established base over $1 NWBO is holdings its gains with ease and surging higher with power as Investors eagerly await top line data from the Phase III trial of DCVax®-L.
Speculation is sky high that NWBO is on the verge of major success where all others have failed including many big names recently who hail DCVax®-L as an effective treatment/cure for the deadliest, most treatment resistant cancers. There have been indications that the top line date for DCVax®-L will be overwhelmingly positive. Glioblastoma multiforme (GBM) represents a potential market expected to reach $1.4 billion by 2025. DCVax®-L has been featured by main stream press numerous times as many recovered patients have come forward crediting DCVax®-L for saving their lives. The stock represents easily among the highest risk-reward opportunities in the market today and if top line data is positive as many speculate it will be, there is no limit to how high NWBO can go. Positive results should provide Linda Powers and her board ample bargaining power to sell NWBO’s proprietary vaccine to the highest bidder, which would readily be in the $10 to $20 billion market cap range.
 NWBO (Northwest Biotherapeutics) is developing cancer vaccines designed to treat a broad range of solid tumor cancers more effectively than current treatments, and without the side effects of chemotherapy drugs. NW Bio’s proprietary manufacturing technology enables the Company to produce its personalized vaccine in an efficient, cost-effective manner. The Company has a broad platform technology for DCVax dendritic cell-based vaccines. NWBO owns a valuable patent portfolio of 199 issued patents and 65 pending patent applications worldwide, grouped into 11 patent families. For a look at NWBO recent history go here. As per their latest quarterly report NWBO had $9.2 million in the treasury. Since then, according to a recent 8k, the company acquired a loan of around $11mn non-dilutive.
NWBO (Northwest Biotherapeutics) is developing cancer vaccines designed to treat a broad range of solid tumor cancers more effectively than current treatments, and without the side effects of chemotherapy drugs. NW Bio’s proprietary manufacturing technology enables the Company to produce its personalized vaccine in an efficient, cost-effective manner. The Company has a broad platform technology for DCVax dendritic cell-based vaccines. NWBO owns a valuable patent portfolio of 199 issued patents and 65 pending patent applications worldwide, grouped into 11 patent families. For a look at NWBO recent history go here. As per their latest quarterly report NWBO had $9.2 million in the treasury. Since then, according to a recent 8k, the company acquired a loan of around $11mn non-dilutive.
 The Company’s lead product, DCVax®-L, is designed to treat solid tumor cancers in which the tumor can be surgically removed. This product is in a 331-patient Phase III trial for newly diagnosed Glioblastome multiforme (GBM). Recently the database for the Phase III trial of DCVax®-L for Gliobastoma was locked. On Oct. 5, NWBO issued a press release detailing many steps that would ensue, including the unblinding of the company’s management of the results of the statistical analysis of the data. “With the database now locked, the independent service firms managing the Clinical Trial are arranging for the independent statisticians to have access to the unblinded raw data from the Trial. Neither the Company nor any party other than the independent statisticians will have access to any unblinded data at this stage. The Phase III trial went on for years as the Company waited for the 233rd death to occur out of 331 patients to calculate whether DCVax-L has any survival benefit. The fact that it took so long is another highly positive pointer here.
The Company’s lead product, DCVax®-L, is designed to treat solid tumor cancers in which the tumor can be surgically removed. This product is in a 331-patient Phase III trial for newly diagnosed Glioblastome multiforme (GBM). Recently the database for the Phase III trial of DCVax®-L for Gliobastoma was locked. On Oct. 5, NWBO issued a press release detailing many steps that would ensue, including the unblinding of the company’s management of the results of the statistical analysis of the data. “With the database now locked, the independent service firms managing the Clinical Trial are arranging for the independent statisticians to have access to the unblinded raw data from the Trial. Neither the Company nor any party other than the independent statisticians will have access to any unblinded data at this stage. The Phase III trial went on for years as the Company waited for the 233rd death to occur out of 331 patients to calculate whether DCVax-L has any survival benefit. The fact that it took so long is another highly positive pointer here.
NWBO is pursuing an intensive program of manufacturing preparations and planning as it approaches top line data from its Phase III trial of DCVax®-L. NWBO is currently building a manufacturing facility in Sawston, Uk. The buildout is the culmination of several years of design, development and preparatory activities, including clean room suites, quarantined storage, quality control testing suites, controlled cryostorage (freezing) facilities for the finished products, as well as specialized systems such as full air changes every 60 seconds in the clean room suites, and precise monitoring of particle counts in the clean room air. This accelerated effort is being supported by the Company’s recent financings and by a special purpose competitive loan of £1.35 (~$1.77) million from the Department for Business, Energy & Industrial Strategy which is administered locally in the Cambridge/Sawston region by the Cambridgeshire & Peterborough Combined Authority. The project cost is approximately $4.6 million. NWBO also recently aquired Flaskworks.
Investor Sentiment in NWVO is very high:
Absolutely we are. $NWBO and their #dcvax is about to change cancer treatment forever.
— Galadon Gaming (@GaladonGaming) March 11, 2021
$nwbo they’re going to cure Glioblastoma brain cancer. We’re waiting on locked in phase 3 data to release.
— AK (@akram1134) March 10, 2021
(2) $NWBO may be working with the 4 regulatory authorities on the pathway to submit a new BLA or MAA for #DCVax. I know that COVID has also caused some issues. We just don’t know for sure. The 2020 10-K should be filed by end of March or shortly after. I hope we get answers soon.
— ATLnsider (@ATLnsider) March 11, 2021
To Find out the inside Scoop on NWBO Subscribe to Microcapdaily.com Right Now by entering your Email in the box below
 There have been indications that the top line date for DCVax®-L will be overwhelmingly positive; according to date published from 2018 at the Society for Neuro-Oncology Annual Meeting in New Orleans shows the following: Median overall survival (OS) from surgery in the intent-to-treat population at data cutoff was 23.1 months, the same OS benefit identified in 2017. The survival rate at year 2 was 46.4%, on par with 46.2% in 2017. In patients with methylated MGMT genes, median OS after surgery was 35.1 months, slightly better than 34.7 months observed in 2017. This represents a vast improvement over the median OS of only 14.6 months. “The survival rate is quite remarkable compared to what would be expected for glioblastoma,” said lead author Dr. Linda Liau, professor of neurosurgery at the David Geffen School of Medicine at UCLA and a member of the UCLA Jonsson Comprehensive Cancer Center. “The 20 to 30 percent of long-term survivors in immunotherapy clinical trials are the people in whom we think there may be a particularly strong immune response against their cancer that is protecting them from getting tumor reoccurrence.”
There have been indications that the top line date for DCVax®-L will be overwhelmingly positive; according to date published from 2018 at the Society for Neuro-Oncology Annual Meeting in New Orleans shows the following: Median overall survival (OS) from surgery in the intent-to-treat population at data cutoff was 23.1 months, the same OS benefit identified in 2017. The survival rate at year 2 was 46.4%, on par with 46.2% in 2017. In patients with methylated MGMT genes, median OS after surgery was 35.1 months, slightly better than 34.7 months observed in 2017. This represents a vast improvement over the median OS of only 14.6 months. “The survival rate is quite remarkable compared to what would be expected for glioblastoma,” said lead author Dr. Linda Liau, professor of neurosurgery at the David Geffen School of Medicine at UCLA and a member of the UCLA Jonsson Comprehensive Cancer Center. “The 20 to 30 percent of long-term survivors in immunotherapy clinical trials are the people in whom we think there may be a particularly strong immune response against their cancer that is protecting them from getting tumor reoccurrence.”
Glioblastoma, also known as glioblastoma multiforme (GBM), is the most aggressive type of cancer that begins within the brain. There is no known method of preventing the cancer. Treatment usually involves surgery, after which chemotherapy and radiation therapy are used. The medication temozolomide is frequently used as part of chemotherapy. High-dose steroids may also be used to help reduce swelling and decrease symptoms. Despite maximum treatment, the cancer usually recurs. The typical duration of survival following diagnosis is 12 to 15 months, with fewer than 3 to 7% of people surviving longer than five years. Without treatment, survival is typically three months. Brain and other nervous system cancers are the 10th leading cause of death for men and women. With the increasing prevalence of brain cancer, rising aging population, and growing incidences of cancer via chemical exposure, the demand of diagnosis and treatment of brain tumors is steadily increasing. The diagnosis, treatments, therapeutics and drug segments are all projected to continue to rise at a significant pace over the next several years. A report from Industry Research concerning the diagnostic and treatment markets projected that the global Brain Tumor Diagnosis and Treatments market size is projected to reach US$ 590 million by 2026, from US$ 444.8 million in 2020, at a CAGR of 4.8% during 2021-2026… while another report from ResearchAndMarkets, focusing on the drugs market added that the global brain tumor drugs market, which was valued at about $2.4 billion in 2018, is expected to grow to $3.41 billion at a CAGR of 9.2% through 2022. One player that has emerged in the space is NovoCure (NVCR), which now markets Optune and Optune Lua, a Tumor Treating Fields delivery system for use as a monotherapy treatment for adult patients with glioblastoma. This treatment has over 3400 active patients, and made $144mn in Q4 revenue this year.
To be published April 28, 2021, 240 pages.
This issue of Neurosurgery Clinics, guest edited by Dr. Linda M. Liau, is dedicated to Glioblastoma: Molecular and Clinical Trials.$nwbo #dcvax #glioblastoma #cancer #vaccine $mrk $bmy $rhhby @UCLAJCCChttps://t.co/QNc9AIkEey pic.twitter.com/ROy4nLeKxw
— rj (@sharpie510) March 6, 2021
For More on NWBO Subscribe Right Now!
Northwest Biotherapeutics, Inc (OTCMKTS: NWBO) has moved back into full beast mode and has been running higher in recent trading on heavy accumulation. NWBO is the darling of small caps and has proven itself as among the most resilient stocks in small caps with a huge following of investors. With a 52-week high of $2.51 and a newly established base over $1 NWBO is holdings its gains with ease and surging higher with power as Investors eagerly await top line data from the Phase III trial of DCVax®-L. Speculation is sky high that NWBO is on the verge of major success where all others have failed including many big names recently who hail DCVax®-L as an effective treatment/cure for the deadliest, most treatment resistant cancers. There have been indications that the top line date for DCVax®-L will be overwhelmingly positive. Glioblastoma multiforme (GBM) represents a potential market expected to reach $1.4 billion by 2025. DCVax®-L has been featured by main stream press numerous times as many recovered patients have come forward crediting DCVax®-L for saving their lives. The stock represents easily among the highest risk-reward opportunities in the market today and if top line data is positive as many speculate it will be, there is no limit to how high NWBO can go. Positive results should provide Linda Powers and her board ample bargaining power to sell NWBO’s proprietary vaccine to the highest bidder, which would readily be in the $10 to $20 billion market cap range. Microcapdaily first reported on NWBO in 2018 and continues today. We will be updating on NWBO when more details emerge so make sure you are subscribed to Microcapdaily so you know what’s going on with NWBO.
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Disclosure: we hold no position in NWBO either long or short and we have not been compensated for this article.
You may like
-


Northwest Biotherapeutics, Inc (OTCMKTS: NWBO) Red Hot as Positive top-line Data in DCVax®-L Phase 3 trial for Glioblastoma is Picked Up by Mainstream Press
-


Northwest Biotherapeutics, Inc (OTCMKTS: NWBO) Major Breakout Northbound as Dr. Linda Liau Set to Give Presentation on Murcidencel at the Society for Neuro-Oncology Conference on Sunday
-


Northwest Biotherapeutics, Inc (OTCMKTS: NWBO) Heats Up as DCVax®-L Proves Safe & Effective While Sawston Facility Applies for Commercial Manufacturing License for Cellular Therapies
-


Northwest Biotherapeutics, Inc (OTCMKTS: NWBO) Looking to Breakout After Phase 3 Clinical Trial of DCVax®-L for GBM Showed Improved Overall Survival & Favorable Safety Profile
-


Northwest Biotherapeutics, Inc (OTCMKTS: NWBO) Coming Back After Major Short Attack While Biotech Reports Primary and Secondary Endpoints Met for DCVax®-L with Excellent Safety Profile
-


Northwest Biotherapeutics, Inc (OTCMKTS: NWBO) Bull Run as Biotech Set to Release Phase III Top Line Data for DCVax®-L May 10 / Dr. Linda Liau Will Present “Autologous Tumor Lysate-Loaded Dendritic Cell Vaccination for Glioblastoma” at the New York Academy of Sciences
Emerging Markets
Bitfarms (NASDAQ: BITF) in Focus: Exploring the Meteoric 110% Surge and Macro Influences
Published
4 months agoon
December 7, 2023
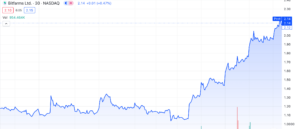
Bitfarms Ltd. (NASDAQ: BITF) rides the wave of explosive growth surging by over 110% from its lows of $1.03 per share in November. This BTC miner has witnessed a 41% increase since last Friday, December 1st, 2023. If you’ve been keeping up with our recent articles, you’ll notice that majority of coverage lacks substantial press releases or filings supporting a surge in valuation. Today’s coverage includes precisely that and more. Let’s delve deeper into BITF to assess if this crypto miner is worth considering amidst the ongoing rise in spot BTC.
 Background:
Background:
Established in 2017, BITF has established itself as a global leader in Bitcoin (BTC) mining. Utilizing its computational power, the company contributes to various mining pools, earning payment in Bitcoin.
BITF sets itself apart by developing, owning, and operating vertically integrated mining farms. These facilities feature in-house management, company-owned electrical engineering services, installation support, and multiple on-site technical repair centers, ensuring a comprehensive operational setup. Bitfarms relies on its proprietary data analytics system, ensuring superior operational performance and uninterrupted service.
Currently managing 11 farms across four countries—Canada, the United States, Paraguay, and Argentina—Bitfarms predominantly uses environmentally friendly hydro-electric power and maintains long-term power contracts. The company remains dedicated to employing sustainable energy sources, often utilizing locally available and under-utilized energy infrastructure.
In its recent upgrade initiative, BITF has improved its fleet’s efficiency and capital practices ahead of the Halving. This strategic move aims to boost its capital-efficient fleet to 12.0 EH/s by Q2 2024. The focus lies in reducing miner and energy costs, enhancing fleet energy efficiency, and fostering greater pricing flexibility for sustained growth and success.
Subscribe to Microcapdaily.com Right Now by entering your Email in the box below.
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Market Update:
At the core of BITF’s latest momentum and strength lies its connection to macro-scale factors. To provide deeper insights into BITF’s future prospects, we’ve conducted a comprehensive market analysis.
The latest market surge propelled spot BTC above the USD $44,000 mark at its peak, reaching its highest value in more than 19 months. This remarkable rally emphasizes the enduring positivity shared among retail and institutional traders alike.
Blackrock ETF:
The primary driving force behind Bitcoin’s ascent appears to be rooted in market expectations of an approved spot BTC exchange-traded fund (ETF) in January 2024. This anticipation has spurred substantial cash inflows from institutional investors, contributing significantly to Bitcoin’s current trajectory.
If the approval unfolds as anticipated, it has the potential to bring a substantial wave of fresh capital into Bitcoin. According to certain projections, this approval might introduce up to $50 billion in new liquidity to Bitcoin. The precise impact on Bitcoin’s price remains uncertain; however, a particular model suggests a 4% price surge for every $1 billion that enters Bitcoin. Consequently, if the projected $50 billion materializes, Bitcoin could potentially double or even triple in value.
Rumours from Qatar:
As per bitcoin enthusiast Max Keiser, Qatar’s sovereign wealth fund (QSWF) (mainly tasked with managing the nation’s extensive oil and gas-derived wealth), is contemplating a significant investment spree of up to $500 billion in the flagship cryptocurrency, Bitcoin.
To offer a comparison, this proposed investment dwarfs the disclosed Bitcoin holdings of MicroStrategy, founded by Michael Saylor, by an astonishing 671 times. Presently, MicroStrategy stands as the largest corporate holder of Bitcoin, possessing 174,530 BTC following its acquisition in November.
Keiser is notably optimistic that QSWF’s monumental investment could propel the price of bitcoin to reach soaring highs of $100,000.
Binance Update:
In the aftermath of former Binance CEO Changpeng Zhao’s guilty plea and the subsequent $4.3 billion settlement with the U.S. Department of Justice (DOJ) on Nov. 21, Bitcoin’s price initially showed mixed signals. Contrary to expectations, Binance did not experience a mass exodus of funds similar to what FTX faced during its public liquidity crisis. Notably, prominent figures in the crypto space, such as Galaxy Digital CEO Mike Novogratz, perceive the settlement as a positive development overall.
At first, Binance’s Bitcoin reserves dropped by 17% from their peak. Following the initial outflows, the balance has shown an increase of nearly 1%. As for FTX, their BTC reserves fell a staggering 99.9% from all-time highs in November 2022 ,with no recovery in sight.
This serves as a significant testament to Bitcoin’s resilience at present, even as the largest cryptocurrency exchange by volume faces a monumental lawsuit. Unlike the substantial impact experienced by FTX, this legal challenge did not severely affect BTC reserves.
So What?:
With multiple factors driving spot BTC, smaller-scale BTC miners are becoming increasingly attractive -especially considering the potential financial growth and rising profit margins. Let’s delve into the recent developments concerning BITF for a closer look.
Subscribe to Microcapdaily.com Right Now by entering your Email in the box below.
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
What Happened:
On December 1st, 2023, BITF released a comprehensive performance and financial update, revealing robust figures for their BTC mining operations. Based on this report alone, it’s evident that BITF boasts a substantial global operational capacity and possesses the financial support needed to remain competitive, particularly as BTC mining grows more challenging post-halving. To enhance clarity, we’ve restructured the release into bullet points for easier comprehension.
BTC Production: November production recorded 392 BTC, a 1.5% decline from October. The network difficulty surged by 19.0% in November, indicating strong miner demand leading into the 2024 Halving.
Network Metrics: For the eleven months ended November 30th, network difficulty increased by 92.2%, while BTC’s price rose approximately 128.4%. This resulted in a 33.9% improvement in production economics measured by USD/TH/day.
Operating Highlights:
- 6.4 EH/s was online by November 30, 2023, marking a 45% increase from November 30, 2022.
- 66.4 BTC/average EH/s was recorded, down 1.9% from October 2023.
- 13.1 BTC earned daily on average in November, equivalent to about $495,200 per day based on a BTC price of $37,800 on November 30, 2023.
Expansion Initiatives:
- Firm purchase order placed for 35,888 Bitmain T21 miners, with an option for an additional 28,000 T21 miners.
- Finalizing contracts to expand operating capacity from 30 MW to 50 MW at Paso Pe, adding 20 MW of hydro-miner containers.
Financial Update:
- Raised $44 million in gross proceeds through a private placement of common stock and warrants.
- Sold 350 BTC, generating $12.8 million in total proceeds.
- Added 42 BTC to treasury, holding a total of 802 BTC, valued at approximately $30.3 million based on a BTC price of $37,800 at November 30, 2023.
- Held Synthetic HODL™ of 35 long-dated BTC call options as of November 30, 2023.
- Reduced outstanding indebtedness by $1.9 million, leaving a remaining balance of $6.0 million at November 30, 2023.
Technical Analysis:
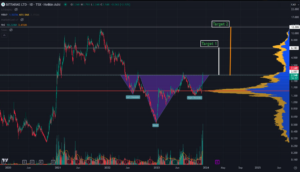
We’re noticing a trend among retail traders on social platforms like X, discussing technical trading patterns related to crypto miners, specifically highlighting positive momentum linked to an Inverse Head and Shoulders (IHS) pattern. A user, @FreeDoomCapital, suggests that among all miners, BITF seems to exhibit the clearest pattern. Here’s a brief explanation of the pattern to enhance your comprehension.
The Inverse Head and Shoulders (IHS) pattern is a technical analysis formation commonly observed in financial markets, particularly in stocks, currencies, and cryptocurrencies. It’s considered a bullish reversal pattern and typically appears after a downtrend.
Here’s a further breakdown on the pattern:
- Formation: The pattern consists of three successive troughs. The middle trough (the “head”) is the lowest point, while the two surrounding troughs (the “shoulders”) are higher than the head and relatively symmetrical in height.
- Shoulders: The left and right shoulders are formed at the end of a downtrend. They show a decline in price followed by a temporary stabilization or slight increase before declining again.
- Head: The head is formed after the left shoulder, indicating a further decrease in price, often reaching a new low. However, the head is typically higher than the previous trough, indicating a potential shift in the downward momentum.
- Neckline: The neckline is a trendline connecting the highs of the two shoulders. It acts as a critical level; a breakout above this line is a significant signal for a potential trend reversal.
- Volume: Volume analysis can complement the pattern. Generally, during the formation of the left shoulder, the volume decreases, increases during the head formation, and decreases again during the right shoulder formation. A breakout with higher volume after the formation is considered a stronger confirmation of the pattern.
- Confirmation: A confirmed Inverse Head and Shoulders pattern occurs when the price breaks above the neckline. Traders often look for a sustained move above the neckline to confirm the reversal.
The Inverse Head and Shoulders pattern is considered complete when the price breaks above the neckline, indicating a shift from a downtrend to a potential uptrend. Traders and analysts use this pattern as a signal to anticipate higher prices, and they often set price targets based on the distance between the neckline and the head.
Similar to all technical trading patterns, it’s important to recognize that it’s not foolproof and may not be accurate in every instance. However, it’s noteworthy that several sources are paying attention, particularly due to the apparent clarity of BITF’s formation of an IHS pattern in their lineup.
Conclusion:
An essential consideration with BTC miners is the increasing difficulty of mining the digital asset after halving, which is scheduled for around April 2024. BITF’s recent fleet upgrade aims to strengthen its capacity pre-halving, positioning itself as a frontrunner. With spot BTC around USD $44,000, the 392 BTC mined in November alone would translate to approximately USD $17 million in top line revenue. If potential BTC catalysts sky rocket the price to USD $150,000 as some suggest, November’s mining performance would represent nearly $60 million – and that’s just in one month. As one of the leading BTC miners globally, we highly recommend keeping BITF on your radar.
We will update you on BITF when more details emerge, subscribe to Microcapdaily to follow along!
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Disclosure: We have not been compensated for this article/video. MicroCap Daily is not an investment advisor; this article/video does not provide investment advice. Always do your research, make your own investment decisions, or consult with your nearest financial advisor. This article/video is not a solicitation or recommendation to buy, sell, or hold securities. This article/video is our opinion, is meant for informational and educational purposes only, and does not provide investment advice. Past performance is not indicative of future performance.
Picture by WorldSpectrum from Pixabay
Emerging Markets
Music Licensing Inc. (OTC: SONG): Unraveling the 1250% Surge and Current Valuation
Published
5 months agoon
November 10, 2023
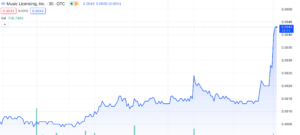
Music Licensing, Inc. (OTC: SONG) shares skyrocket a whopping 100% gain at the time of writing, marking an incredible 1250% surge since their October low of $0.0004. Many appear to be wondering the cause behind the sudden rise and after today’s spike we decided to do more research. Let’s dig into the company and its recent updates to figure out how they’ve pulled off such an impressive climb and what might be coming next.
Background:
Starting with their name – Music Licensing, Inc., it is more commonly recognized as Pro Music Rights. If you’re diving into your own research, you’ll likely want to search by, “Pro Music Rights” to find the investor information you’re looking for, you won’t find much under the stock name alone.
In a nutshell, SONG is a performance rights organization (PRO) and was the 5th to be established in the United States. If that mean’s absolutely nothing to you, skip to the next title for a better understanding.
Nethertheless, they’ve got some big players under their license umbrella, including TikTok, iHeart Media, Triller, Napster, 7Digital, and Vevo. In total, SONG holds an estimated market share of 7.4% in the United States, representing a whopping 2,500,000 works.
Look out for familiar artists like A$AP Rocky, Wiz Khalifa, and Young Jeezy in their impressive lineup. For more details on other notable artists, their press releases have a comprehensive list for you to explore.
Again if the whole idea of a Performance Rights Organization (PRO) feels confusing, you’re not alone. We did some digging to break it down for you.
What’s a Performance Rights Organization:
To keep it simple, think of a Performance Rights Organization (PRO) as the backstage manager for musicians. It’s like the middle person making sure that when your favorite songs are played in public—on the radio, at concerts, or even on your go-to streaming app—the artists get their fair share of the spotlight. These organizations, such as ASCAP, BMI, and SESAC, keep tabs on where and how music is being enjoyed and make sure the right people get paid for their tunes. It’s a behind-the-scenes gig that ensures artists get a nod and a paycheck every time their music takes center stage.
Latest Press Release:
This morning on November 10th, 2023, the company announced it will be cancelling 59.9% of their outstanding shares to enhancing Shareholder Value.
“Following the cancellation of an astounding 1,566,945,290 common stock shares, which reduced the total outstanding shares to 2,000,000,000, Mr. Noch is now embarking on yet another groundbreaking endeavor. He has pledged to cancel an additional 1,197,364,785 common stock shares, equating to a remarkable 59.9% of the current outstanding shares”.
Taking the cancellation of all these shares would also mean their market cap would be effected pretty drastically as well. If we do the math (at time of writing), 2B shares outstanding multiplied by the current stock price of $0.004 would mean ~8M market cap.
Okay Jake, so you’ve done the share reduction dance, claiming it’s a boost for shareholders. But really, what else does cutting down on shares mean for investors. Allow us to further break it down.
Subscribe to Microcapdaily.com Right Now by entering your Email in the box below.
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Why Does it Matter?
Cutting down on the number of shares a company has floating around might seem like financial gymnastics, but it actually makes sense for a few reasons:
- More Bang for Your Buck (EPS): Picture this – you and your buddies own a pizza joint. If you slice up the pizza into more pieces, each one gets smaller. The same goes for shares. Less shares mean each piece of the company’s earnings pie goes to fewer shareholders, so everyone’s slice gets bigger. That’s what we call Earnings Per Share (EPS), and a bigger slice is usually good news.
- Share Price Perk: When a company trims down its shares, it can potentially give its stock price a boost because the float is now tighter. With a tighter float, it doesn’t take as much buying to move the stock price in either direction.
- Steady Ship: Fewer shares mean fewer folks holding the reins. It’s like steering a ship – with fewer hands on deck, it’s easier to keep things steady. Reducing the number of shares available for purchase also makes it harder and more costly for others to buy a big chunk of ownership, fortifying the company against hostile takeovers.
- Improved Financial Ratios: Reducing shares outstanding can even positively impact financial ratios, such as earnings per share, return on equity, and book value per share. These improvements of course make their financial profile more attractive to investors by and large.
Financial Highlights:
Despite having a market cap of around $15 million on YahooFinance, or $8 million if we consider the share consolidation, SONG has posted impressive numbers for its financials. They’ve managed to rake in a substantial $758 million in revenues, pushing a solid net income of $39 million. Their quarter ending in June, 2023 showed 93M in revenue, which was also net profitable too. Digging into their balance sheet, you’ll find $45 million in assets and a modest $61,000 in liabilities. Given those figures, this valuation’s definitely a head-scratcher and seems completely off.
Subscribe to Microcapdaily.com Right Now by entering your Email in the box below.
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Behind SONG’s Low Valuation:
According to one user, @StockPicksNYC, on Twitter there’s a story behind why their valuation is so low given their fundamental financial backing.
Apparently the stock’s low valuation can be attributed to a previous incident where a storm-induced power outage at the CEO’s residence led the company to a downgrade on OTC markets, pushing SONG into ‘Expert Market‘ status with a ‘Shell Risk’ label.
Despite successfully addressing challenges to regain ‘Pink Current’ status and remove the ‘Shell Risk’ designation, SONG is still unable to update its information on the OTCM platform.
This is a well-documented problem with OTCM and there’s ongoing litigation with OTC Link, (an OTC Markets subsidiary), revolving around transparency and responsiveness issues during efforts to resolve downgrade-related issues.
This has now pushed SONG to take further measures to safeguard itself and its shareholders. They currently have a lawsuit filed against OTC Markets, where they’re pursuing $386.6 Million in Damages.
The good news is despite the lack of information on OTCM, SONG filed a Form 1-SA with the SEC to provide detailed information about their business.
OTC Expert Market:
If we look at the OTC Market tiers, there’s OTCQX, OTCQB, OTCPNK, OTC Expert Market, and OTC Grey. Back on September 28, 2021 the SEC put an amendment in place that stops brokers from quoting stocks without current information. That’s where OTC’s Expert Market steps in.
In a nut shell, any company that does not have current information publicly available trades on OTC Expert Market. Only broker-dealers, professionals or sophisticated investors are allowed to view quotations in Expert Market securities. It of course comes with massive risk given you’d be completely unaware of the company’s financial health.
Conclusion:
Among many factors, SONG’s impressive revenue and profits alone make the company appear considerably undervalued. Even without more developments, we can imagine a company of this caliber will inevitably draw increasing interest from investors. Considering the legal issues with OTC, a move to a bigger exchange like the NYSE or NASDAQ seems likely. They’d atleast be a great candidate considering the fundamentals. Keep in mind, this dynamic story could change at a moments notice, so be sure to keep them on your radar.
We will update you on SONG when more details emerge, subscribe to Microcapdaily to follow along!
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Disclosure: We have not been compensated for this article/video. MicroCap Daily is not an investment advisor; this article/video does not provide investment advice. Always do your research, make your own investment decisions, or consult with your nearest financial advisor. This article/video is not a solicitation or recommendation to buy, sell, or hold securities. This article/video is our opinion, is meant for informational and educational purposes only, and does not provide investment advice. Past performance is not indicative of future performance.
Picture by whoalice-moore from Pixabay
Emerging Markets
Lucy Scientific Discovery’s (NASDAQ: LSDI) Game-Changing Move: A Closer Look at the High Times Acquisition
Published
7 months agoon
September 8, 2023
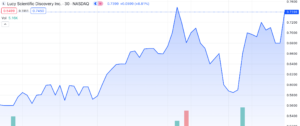
On August 8th, 2023, Lucy Scientific Discovery Inc. (NASDAQ: LSDI), a leading developer in the psychedelic drug industry, witnessed an impressive surge in its stock value, gaining approximately 25% in combined trading, including after-hours (AH) trading. The British Columbia-based company made headlines by announcing its strategic move to acquire intellectual property (IP) from the renowned cannabis publication, High Times Holding Corp. (HHC).
Additional Background:
Under this agreement, Lucy will exchange 20% of its shares and a series of payments for access to HHC’s valuable IP portfolio, which includes the rights to generate licensing and royalty income from renowned brands like High Times, 420.com, and Cannabis Cup, along with their associated domain names.
The partnership between #HighTimes and #LucyScientificDiscovery just makes sense. It's a harmonious alignment that should lead to success. This could be the start of something special as they journey towards ambitious goals.$LSDI $TSLA $SPY pic.twitter.com/qgXYkKQVoe
— Luca Grayson (@GraysonLucasa) September 7, 2023
Lucy’s commitment involves making semi-annual payments to HHC over a five-year period, structured around earnings before income, taxes, depreciation, and amortization (EBITDA) generated through the acquired IP. The flexibility exists for Lucy to fulfill these payments either in cash or through stock issuance and the announcement is generating considerable interest.
Furthermore, post-acquisition, Lucy will grant High Times the opportunity to operate retail outlets and distribute THC products bearing these prestigious brands within the United States. This privilege comes in exchange for an annual license fee of $1 million, set to double to $2 million annually once federal legalization of cannabis occurs in the country.
Subscribe to Microcapdaily.com Right Now by entering your Email in the box below.
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Leveraging the brand rights secured from HHC, Lucy aims to bolster its revenue streams by expanding and enhancing its existing 18 licensing agreements, both domestically and internationally. These arrangements encompass a wide array of consumer products and merchandise, promising to further establish Lucy’s presence in the global market. The acquisition is expected to be finalized within the coming two weeks, marking a significant strategic move for Lucy Scientific Discovery Inc.
As a result of the acquisition, High Times is now a publicly-traded entity. Lucy anticipates that this agreement will contribute over $10 million in revenue to its financial results in the upcoming year, along with $5 million in EBITDA.
By purchasing #HighTimes assets, Lucy Scientific Discovery has struck gold. It has acquired a wealth of intellectual property, trademarks, and royalty prospects that will bring #LSDI $LSDI #lucyscientificdiscovery millions of dollars. pic.twitter.com/CEkqQnuFHm
— Rowan Dune (@rowandune) September 7, 2023
Adam Levin, the Executive Chairman of HHC, expressed optimism about the deal, noting, “This transaction will create exciting new growth opportunities for the High Times brand, under the leadership of Richard Nanula, a seasoned executive with extensive experience in major consumer brands and global corporations.”
Levin also emphasized High Times’ enthusiasm in becoming a significant shareholder of Lucy Scientific Discovery. Notably, Lucy completed its initial public offering and Nasdaq listing in February, offering 1,875,000 shares at $4.00 each.
Richard Nanula, CEO of the British Columbia-based company, shared his outlook on the acquisition, stating, “Lucy expects this acquisition to rapidly generate high-margin revenue within the global cannabis sector.”
In recent developments, Lucy introduced the sleep aid product “Twilight,” which includes amanita muscaria and reishi mushrooms. Additionally, the company joined forces with Wesana Health Holdings Inc. (OTCQB: WSNAF) in March to collaborate on the development of the CBD and psilocybin-based drug SANA-013, targeting conditions such as migraines, cluster headaches, and major depressive disorder.
$LSDI's Health Canada license allows approved psilocybin sales, backed by the Canadian government's significant funding $LSDI is on fire. #LucyScientificDiscovery $LSDI
— William James (@jaym_willy) September 8, 2023
High Times, founded in 1974, has a rich history, featuring works by renowned writers like Truman Capote and Hunter S. Thompson. Since 1988, its Cannabis Cup has stood as the most prestigious cannabis competition globally, with notable judges including Snoop Dogg, Joe Rogan, Tommy Chong, and other prominent figures in the cannabis industry.
While Lucy’s shares showed a nearly 16% increase to reach $0.68 on the Nasdaq exchange on Friday, it is worth noting that they have experienced a decline of over 77% over the past year.
Macro Trend:
In recent times, our articles have prominently featured cannabis-related topics, reflecting the growing popularity of stocks in this sector. LSDI’s acquisition aligns perfectly with the current climate, as the cannabis industry experiences a significant surge, coinciding with the Health and Human Services (HHS) exploring the possibility of reclassifying cannabis from Schedule I to Schedule III of the Controlled Substances Act.
Don't overreact to temporary $LSDI EPS decline. Psychedelics mega-trend makes risk/reward compelling.#Shrooms#LucyScientificDiscovery
— gianna snell (@skatette) September 8, 2023
While many countries around the world have already moved towards decriminalization and legalization, the United States has been relatively cautious in its approach. However, the consideration of such a reclassification represents a potential historic turning point. If such a change were to materialize, it would mark a substantial shift in the regulatory landscape, potentially revitalizing cannabis as an attractive investment opportunity. The industry is already showing signs of reestablishing its market presence and could once again become a noteworthy investment option.
I know this Mindful line is set to disrupt the market. Expecting a surge in stock price in the months ahead. #Lucyscientificdiscovery $LSDI pic.twitter.com/2ccR1CttA3
— Tom (@Tomi_l33) September 7, 2023
We will update you on LSDI when more details emerge, subscribe to Microcapdaily to follow along!
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Disclosure: We have not been compensated for this article/video. MicroCap Daily is not an investment advisor; this article/video does not provide investment advice. Always do your research, make your own investment decisions, or consult with your nearest financial advisor. This article/video is not a solicitation or recommendation to buy, sell, or hold securities. This article/video is our opinion, is meant for informational and educational purposes only, and does not provide investment advice. Past performance is not indicative of future performance.
Picture by herbalhemp from Pixabay
Recent Posts

Clean Vision Corp (OTC: CLNV): Overcoming the Plastic Waste Crisis

Meta Materials (NASDAQ: MMAT): More Due Diligence and Exploring Latest Developments

Integrated Cannabis Solutions’ (OTC: IGPK) 633% Surge: Exploring Catalysts, Company Overview, and Growth Potential in 2024

Sonoma Pharmaceuticals (NASDAQ: SNOA): Potential Surge to Speculations – What Lies Ahead?

1847 Holdings (NYSE: EFSH) Soars: Insights, Acquisitions, and What Lies Ahead
Trending
-

 Uncategorized1 year ago
Uncategorized1 year agoMeta Materials Inc (OTCMKTS: MMTLP) Enormous Short Position in Trouble as Next Bridge Hydrocarbons Set to Stop Trading (George Palikaras & John Brda on Corporate Action)
-

 Micro Cap Insider3 years ago
Micro Cap Insider3 years agoMedium (KOK PLAY) The Parabolic Rise of Metal Arts (OTCMKTS: MTRT)
-

 Media & Technology3 years ago
Media & Technology3 years agoHealthier Choices Management Corp. (OTCMKTS: HCMC) Powerful Comeback Brewing as PMI Patent Infringement Lawsuit Moves Forward
-
Media & Technology3 years ago
AMECA Mining RM; the Rise of Southcorp Capital, Inc. (OTCMKTS: STHC)
-

 Media & Technology3 years ago
Media & Technology3 years agoSNPW (Sun Pacific Holding Corp) Power Brewing: 50MW solar farm project in Durango Mexico MOU with Atlas Medrecycler 48,000 SF New Partnership Queensland Australia Solar Farm.
-

 BioPharma2 years ago
BioPharma2 years agoAsia Broadband (OTCMKTS: AABB) On the Move Northbound Since Sub $0.08 Dip as Crypto Innovator Elevates AABB Crypto Exchange & Enters the NFT Space
-

 Uncategorized1 year ago
Uncategorized1 year agoMeta Materials Inc (OTCMKTS: MMTLP) Short Squeeze S-1a4 Filing Signals S1 Approval Could Be Days Away (Next Bridge Hydrocarbons Spin-Off)
-
 BioPharma2 years ago
BioPharma2 years agoHumbl Inc (OTCMKTS: HMBL) Major Reversal as Powerful Advisor Rejoins the Team & Looks to Uplist to Major Exchange