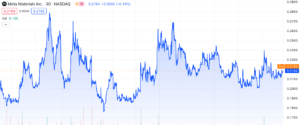
Meta Materials (NASDAQ: MMAT) has been a hot topic as of late, with investors all over the web talking about a potential resurgence. If we rewind to late 2020 and glance at their stock chart, we witness an impressive surge from ~$0.54 to a peak of $13.52, an astonishing 2400% gain within’ the span of a few months. If you’ve been following our articles lately, you’ll notice a similar kind of performance from Tempest Therapeutics’ (NASDAQ: TPST). This is of course a rare event, but there’s a noteworthy angle to consider. While TPST’s initial data release triggered a significant surge, what propelled it further appears to be its “Poison pill” strategy. Recent tweets from MMAT’s CEO suggest a similar strategy is in the works. Could MMAT experience a colossal gain reminiscent of 2021 or even rival TPST’s performance? Let’s delve into Meta Materials, its recent developments, and potential prospects to uncover what’s in store.
Background:
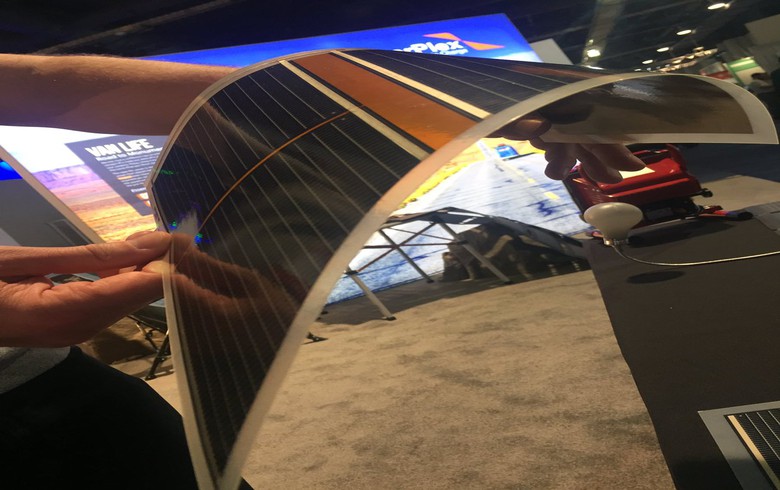
Meta Materials stands at the forefront of advanced materials and nanotechnology. Their focus is on pioneering novel products and technologies utilizing sustainable and innovative scientific approaches. The interesting part is their advanced materials have the transformative power to enhance common products, infusing them with heightened intelligence and sustainability. Leveraging its technology platforms, they’re capable of empowering global brands in creating cutting-edge products that elevate overall performance. Their technology has application across multiple industries including aerospace and defense, consumer electronics, 5G communications, batteries, authentication, automotive, and clean energy. Overall, that’s ~$32B TAM and with current growth rates, it’ll increase to a whopping ~$61B TAM by 2026. Their goal is to shape a smarter and more sustainable world. If you look through their presentation, there are a number of ways their technology can transform our everyday lives. We highly suggest you take a look.
Subscribe to Microcapdaily.com Right Now by entering your Email in the box below.
Lawsuits:
You’ll notice MMAT has faced a challenging year as its valuation took a hit following the initiation of two separate class action lawsuits that stemmed from a short-seller report and statements related to Meta’s business combination with Torchlight Energy Resources.
We’ll keep this section short and focus on the accusations related to the business combination. If you’d like more information on the short seller lawsuit, click here.
Long story short, a shareholder filed a class action lawsuit against Meta on behalf of individuals who acquired the company’s publicly traded securities between September 20, 2020, and December 14, 2021. The lawsuit alleged violations of the Securities Exchange Act of 1934. The complaint outlined that Meta Materials, initially known as Torchlight Energy Resources, Inc., exaggerated its business connections, product capabilities, and pricing during its merger with Metamaterial Inc. The filing highlighted a subsequent SEC subpoena, leading to a share price drop. Additionally, a critical report by Kerrisdale Capital triggered another significant share price decline, further impacting investors.
However 11 days ago on October 2nd, 2023, there were significant positive developments regarding this situation. It appears that MMAT will no longer have this legal burden to bear. The lawsuits were entirely dropped, and the court ruled to dismiss all the allegations made against them. As you might of noticed, the initial announcement earlier this year led to a huge selloff. At the current moment, it’s trading at extremely low levels and many online believe there’s substantial upside.
Poison Pill:
As we previously mentioned, it appears the CEO, George Palikaras is working on a poison pill of his own. After Tempest Therapeutics (NASDAQ: TPST) released their latest data it brought ~2400% gain, but their poison pill managed to push that gain even further to ~4000%. If you’re not familiar with what a poison pill is, allow us to explain below.
A poison pill is a defensive strategy used by a company’s management to deter or prevent hostile takeovers or acquisitions by another entity. The term “poison pill” implies that it is intended to be unattractive or undesirable for the acquiring entity.
Typically, a poison pill involves issuing new shares or other financial instruments to existing shareholders, or allowing them to purchase shares at a significant discount, in the event that an outside entity acquires a certain percentage of the company’s shares. This dilutes the ownership and voting power of the acquiring entity, making the takeover more difficult or costly.
The objective is to make the acquisition financially less appealing or more difficult, encouraging potential acquirers to negotiate with the company’s board of directors instead of pursuing a hostile takeover.
Palikara just recently tweeted, “Revenue, strategic partnerships, cost efficiencies, hiring & paying for performance, non-dilutive capital, poison pill, relentless work, Revenue… Plenty of time 2 get in compliance, but our bar is set a lot higher than that.”
If you look through some of MMAT’s latest releases, you’ll notice they’ve announced various forms of funding, more recently they closed a financing for 50M with Lincoln Park Capital Fund, LLC.
Subscribe to Microcapdaily.com Right Now by entering your Email in the box below.
Potential Application & Outlook:
Pay special attention to this section, as we’ll be spotlighting potential applications of MMAT’s technology and where they’re at in the commercialization process. Many believe the application alone could hold substantial returns for long-term shareholders.
One user from Twitter, @Seashellpants, has shared a video that outlines in great detail MMAT’s agreement with the Simon Fraser university, one of the top universities in Canada and worldwide.
In this video, we’ll catch a glimpse of how the R&D process is going so far and potential application across various verticals. You’ll need to be cautious, as this video may cause “Heavy breathing”.
We’ll provide a brief summary below, but don’t miss out on the hyperlink above. It’s not only entertaining, but also packed with valuable insights.
Breakdown of the Video:
Just over 2 years ago on October 5th, 2021 MMAT acquired Nanotech Security Corp. which is now considered a subsidiary of MMAT. If we delve into MMAT’s 10Q from May 12th, 2023, there are multiple updates on how their research is going with the Simon Fraser University. Within this 10Q we also find an interview with the CTO, Clint Landrock, who unveils numerous case studies related to their nano-manufacturing commercialization efforts.
First and foremost, MMAT has been granted a parent-patent that includes it’s claim for nano-hole structures and applications for those features in the security and authentication industry. It also includes claims for the use of those nano-scale structures that are smaller than a wavelength of light in conjunction with printable electronic components, which would include electronic displays, batteries and solar cells.
Landrock states,”It seems like it could be used for a range of possible markets, including games and interactive displays for consumer products”. He even touches on how these displays could be used for specific light wave optical guides used in medical programs for sensing bacteria and disease or for drug application.
If we delve deeper, the initial purpose of this technology was to enhance solar panels by maximizing electron production, leading to more efficient and durable batteries. Considering their nano-scale structures are tinier than a wavelength of light, you can envision the implications for battery performance. Especially in the context of the ongoing global shift towards Electric Vehicles (EVs), this presents a significant opportunity to integrate such a groundbreaking technology.
However, given the immense demand for this technology across various applications, achieving scalability is critical, necessitating a roll-to-roll manufacturing approach to handle the high volume needed. Typically, scaling up can pose a significant hurdle, but what amplifies the excitement here is Landrock’s affirmation that they have effectively demonstrated, in collaboration with a third party, the ability to produce and operate their technology using high-speed roll-to-roll casting machines. The outcomes were remarkably positive with 100% through-put yield. Which means 100% of the images produced could be used for commercial purposes.
Another common barrier to entry is the costs associated with scaling a new technology. To make things even better, their technology also aligns with global initiative to be more green. Landrock states, “Also, this is a true green technology that will not harm the environment, costs less to produce than the current technology and provides far improved security for authentication requirements”.
Subscribe to Microcapdaily.com Right Now by entering your Email in the box below.
Agreement with Panasonic:
On September 29th, 2023 MMAT teamed up with Panasonic Industry Co. (An operating company in charge of device business within the Panasonic Group) to advance transparent conductive materials. This collaboration aims to enhance the supply of NANOWEB® films, which would benefit sectors like automotive and consumer electronics, transparent film antennas, heaters, and electromagnetic shielding.
The demand for ultra-low sheet resistance and high optical performance is increasing, particularly for flexible solar cells and smart windows. According to BCC Research, the global transparent conductive films market is projected to reach $7.6 billion by 2025 from $4.9 billion in 2020, growing at a CAGR of 9.2%.
George Palikaras, CEO of META, highlighted the importance of this collaboration, emphasizing their shared goal to advance transparent conductive materials. Panasonic Industry has a track record of mass-producing quality transparent conductive films, making them a strategic partner for META.
Yuichi Yoshikawa, Director of Touch Solutions Business Unit at Panasonic Industry, expressed excitement about the collaboration, foreseeing it providing advanced solutions and creating new possibilities across various applications.
This collaboration merges NANOWEB® metal mesh designs by META with Panasonic Industry’s cutting-edge process technology, aiming to set new industry standards. They will showcase their collaborative solutions at CEATEC 2023 which goes from Oct 17 – Oct 20 to demonstrate the potential applications of this partnership.
This agreement holds significant weight. Keep a vigilant watch, the event is around the corner and they’re expecting ~200,000 attendees. A collaboration with a well-established and reputable name like Panasonic certainly changes the landscape, and could bring notable shifts for the company in the near future.
Conclusion:
In essence, MMAT stands at a pivotal moment. With the resolution of lawsuits, it appears things could be looking up. Coupled with their recent strategic maneuvers and advancements in commercialization, MMAT certainly holds promise. Could they potentially see a significant valuation upswing in the near term? The consensus among thousands online is yes. Considering the innovation and potential impact, MMAT is undeniably a company worth vigilant monitoring in the months ahead.
We will update you on MMAT when more details emerge, subscribe to Microcapdaily to follow along!
Disclosure: We have not been compensated for this article/video. MicroCap Daily is not an investment advisor; this article/video does not provide investment advice. Always do your research, make your own investment decisions, or consult with your nearest financial advisor. This article/video is not a solicitation or recommendation to buy, sell, or hold securities. This article/video is our opinion, is meant for informational and educational purposes only, and does not provide investment advice. Past performance is not indicative of future performance.
Picture by geralt from Pixabay