The Tel Aviv Sourasky Medical Center granted its final approval to use SCI-110 in a clinical trial
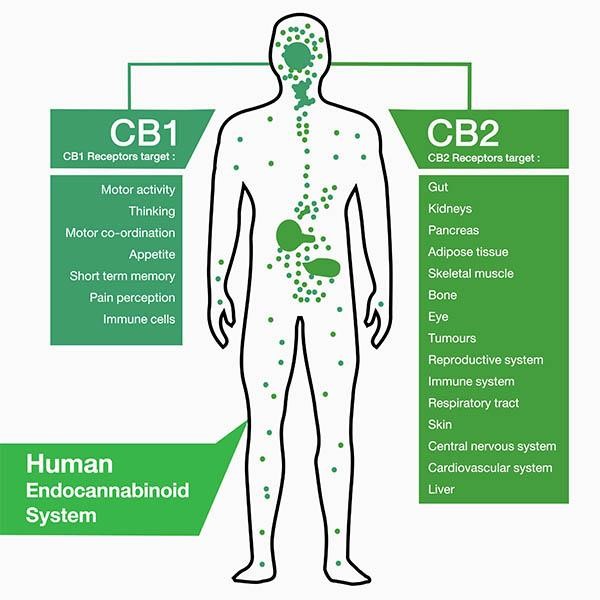
TEL AVIV, Israel, April 04, 2023 (GLOBE NEWSWIRE) — SciSparc Ltd. (Nasdaq: SPRC) (the “Company” or “SciSparc”), a specialty, clinical-stage pharmaceutical company focusing on the development of therapies to treat disorders of the central nervous system, today announced it has been granted final approval from the Tel Aviv Sourasky Medical Center, Tel Aviv, Israel, required to commence the Company’s Phase IIb Clinical Trial for SCI-110 to treat Tourette Syndrome (“TS”).
The Trial is titled “A randomized, double-blind, placebo controlled, cross-over study to evaluate the efficacy, safety and tolerability of daily oral SCI-110 in treating adults with Tourette Syndrome”.
The first clinical site to receive approval to initiate the trial is the Tel Aviv Sourasky Medical Center, Tel Aviv, Israel, under the leadership of Prof. Tanya Gurevich, Head of Movement Disorders Unit, Department of Neurology.
The Company also intends to conduct the clinical trial in various sites including Yale Child Study Center, Yale School of Medicine, Connecticut, USA, under the leadership of Dr. Michael H. Bloch and Hannover Medical School, Hannover, Germany, under the leadership of Prof. Muller-Vahl, Department of Psychiatry. The launch of the clinical trial at Yale University is subject to approvals by the United States Food and Drug Administration and Yale University’s institutional review board and the launch of the clinical trial at Hannover Medical School is subject to approvals by the German Federal Institute for Drugs and Medical Devices.
“We are thrilled to launch our Phase IIb Clinical Trial in Israel and start enrollment of patients suffering from Tourette Syndrome. This trial is a key milestone in SciSparc’s pursuit of lower-dose, THC-based treatment that is potentially safer and more effective for patients than all the current available treatments,” said Oz Adler, Chief Executive Officer of SciSparc.
“TS is estimated to affect 0.5-1% of the world’s population, however the very few available treatments have limited efficacy and questionable safety. Based on previous results from our phase IIa trial conducted at Yale University, we believe our proprietary SCI-110 treatment has the potential to help TS patients around the world,” Adler added.
The objective of this clinical trial is to evaluate the efficacy, safety and tolerability of SciSparc’s proprietary drug candidate SCI-110 in adult patients (between 18 and 65 years of age) using daily oral treatment. The patients will be randomized in a 1:1 ratio to receive either SCI-110 or a SCI-110 matched placebo. The primary efficacy objective of the study will be to assess tic severity change using the Yale Global Tic Severity Scale, the most commonly used measure in clinical trials, as a continuous endpoint at week 12 and week 26 of the double-blind phase compared to baseline. The primary safety objective of the study will be to assess absolute and relative frequencies of serious adverse events for the entire population and separately for the SCI-110 and placebo groups.
SciSparc completes the sale of a 49% interest in its Subsidiary that owns Wellution for $3 million
Upon the closing which occurred on March 22, 2023, the purchase price was adjusted from $2.5 million and increased to approximately $3 million.
TEL AVIV, Israel, March 28, 2023 (GLOBE NEWSWIRE) — SciSparc Ltd. (Nasdaq: SPRC) (“Company” or “SciSparc”), a specialty clinical-stage pharmaceutical company focusing on the development of therapies to treat disorders of the central nervous system, today announced the closing of the definitive agreements for the sale of approximately a 49% equity interest in its wholly owned subsidiary, SciSparc Nutraceuticals Inc. (the “Subsidiary”), which owns Wellution TM , a top-selling Amazon.com Marketplace brand, to Jeffs’ Brands Holdings Inc., a wholly-owned subsidiary of Jeffs’ Brands Ltd. (“Jeffs’ Brands”)(Nasdaq: JFBR), a data-driven e-commerce company operating on Amazon, for $2.5 million in cash and additional deferred cash payments of approximately $489,330 accounting for price adjustments related to inventory and working capital, which will be paid in five equal monthly installments beginning in May 2023 (the “Price Adjustment”), pursuant to the stock purchase agreement dated February 23, 2023 by and between Jeffs’ Brands, Jeffs’ Brands Holdings Inc. and SciSparc. As collateral for the payment in full of the Price Adjustment, SciSparc will hold back such number of shares of common stock of its Subsidiary, equal to the outstanding due amount of the Price Adjustment.
In addition, in connection with the closing, SciSparc and Jeffs’ Brands, will undertake a mutual share exchange in the amount of $288,238 of ordinary shares from each of SciSparc and Jeffs’ Brands.
The number of shares in the share exchange was calculated based on the average closing price of the relevant company’s shares for 30 consecutive trading days ending on the third trading day immediately prior to the closing. Accordingly, SciSparc will acquire 247,415 ordinary shares of Jeffs’ Brands and Jeffs’ Brands will acquire 360,297 ordinary shares of SciSparc having an aggregate value of $288,238, which was adjusted from $300,000 according to the 4.99% ownership limit included in the definitive agreements (the “Exchange Shares”). Following the closing of the transaction which included an equity conversion of financing amounts previously provided to the Subsidiary by SciSparc, and upon satisfaction of the payment in full by Jeffs’ Brands of the Price Adjustment amount, SciSparc will hold approximately 51% of the Subsidiary.
As part of the definitive agreements, at the closing, Jeffs’ Brands and the Subsidiary entered into a consulting agreement by which Jeffs’ Brands will provide management services for Wellution for a monthly fee of $20,000; in addition, Jeffs’ Brands will receive a signing bonus of $51,000. The consulting agreement is for an undefined period and may be terminated by either party with 30-days’ advance notice.
Wellution™ sells dozens of hemp-based, top-ranked products, including hemp gummies, hemp oil capsules, hemp gel, hemp cream, detox pills, height pills, antibacterial creams, and anti-aging creams, among other beauty and hair treatment products that are all manufactured in the United States.
Wellution™ offers eight variations of natural hemp candy supplements under two parent Amazon Standard Identification Numbers (“ASIN”) on Amazon that are differentiated by their hemp oil potency. The leading parent ASIN, that was launched in 2019, has received over 26,500 reviews and is consistently ranked as the #1 best seller in the category. In total, the brand has approximately 40,000 product reviews, most of which are 4 and 5-star reviews.
Mr. Oz Adler, the Chief Executive Officer and the Chief Financial Officer of the Company, is the Chairman of the board of directors of Jeffs’ Brands. The Chairman of the Company, Mr. Amitay Weiss, and Mr. Moshe Revach are members of the board of directors of both SciSparc and Jeffs’ Brands.
Neither the Subsidiary’s shares nor the Exchange Shares have been registered under the Securities Act of 1933, as amended (the “Securities Act”), or any states’ securities laws and may not be offered or sold in the United States, except pursuant to an effective registration statement or an applicable exemption from the registration requirements of the Securities Act.

 OUR NEW PROFILE IS: (
OUR NEW PROFILE IS: (
:max_bytes(150000):strip_icc()/brain_spinal_cord-57fe96b15f9b5805c26d5072.jpg)