SILO Profile
OUR NEW PROFILE IS: (NASDAQ: SILO)
Recently SILO announced its topically administered Ketamine reached a positive end point in an animal study
SILO uplisted to Nasdaq on September 27 and completed a $5.7 million public offering
SILO has $4.6 million in the treasury as of March 31
SILO has a float of just 2.67 Million
_______________________
Hello Everyone,
Turn your attention to SILO right away for Monday’s session.
Silo Pharma Inc (NASDAQ: SILO) is a developmental stage biopharmaceutical company focused on merging traditional therapeutics with psychedelic research. Silo is committed to developing innovative solutions to address conditions such as post-traumatic stress disorder (PTSD), fibromyalgia, Alzheimer’s disease, Parkinson’s disease, and other rare neurological disorders. The company works to identify and partner with leading medical universities, providing the needed financial resources to develop safe therapeutic treatments while moving cutting-edge research through the clinical stage and into commercialization.
Intellectual property: To date, Silo has filed 4 provisional patent applications related to the use of the central nervous system-homing peptides covered by the UMB Option Agreement to deliver certain compounds, including a nonsteroidal anti-inflammatory drug and/or psilocybin, for the treatment of arthritis, central nervous system diseases, neuroinflammatory diseases as well as cancer. In addition, pursuant to the Company’s acquisition of NFID, Silo acquired three trademarks related to the NFID brand.
SILO is Harnessing the Power of Psychedelics to Deliver Breakthrough Medical Solutions
Silo has entered into research agreements and partnerships with multiple leading medical universities, including:
- A sponsored study with Maastricht University utilizing repeated low doses of ketamine and psilocybin to examine the effects on cognitive and emotional dysfunctions in Parkinson’s disease;
- A scientific research agreement with the University of California San Francisco (UCSF) leveraging four other clinical trials being planned by the university to determine the effects of psilocybin on inflammation;
- An exclusive license agreement with the University of Maryland, Baltimore (UMB) to explore a patent focused on enhanced targeting of therapeutic agents to the central nervous system;
- A second exclusive option agreement with UMB to license a joint-homing peptide targeted at arthritis-inflamed joints; and
- An agreement with Columbia University granting it an option to license two distinct assets currently under development, one focused on Alzheimer’s disease and a second focused on PTSD and stress.
Psilocybin is considered a serotonergic hallucinogen and is an active ingredient in some species of mushrooms. Recent industry studies using psychedelics, such as psilocybin, have been promising, and Silo Pharma believes there is a large unmet need with many people suffering from depression, mental health issues and neurological disorders. While classified as a Schedule I substance under the Controlled Substances Act (“CSA”), there is an accumulating body of evidence that psilocybin may have beneficial effects on depression and other mental health conditions. Therefore, the U.S. Food and Drug Administration (“FDA”) and U.S. Drug Enforcement Agency (“DEA”) have permitted the use of psilocybin in clinical studies for the treatment of a range of psychiatric conditions.
The potential of psilocybin therapy in mental health conditions has been demonstrated in a number of academic-sponsored studies over the last decade. In these early studies, it was observed that psilocybin therapy provided rapid reductions in depression symptoms after a single high dose, with antidepressant effects lasting for up to at least six months for a number of patients. These studies assessed symptoms related to depression and anxiety through a number of widely used and validated scales. The data generated by these studies suggest that psilocybin is generally well-tolerated and has the potential to treat depression when administered with psychological support.
Silo Pharma has engaged in discussions with a number of world-renowned educational institutions and advisors regarding potential opportunities and have formed a scientific advisory board that is intended to help advise management regarding potential acquisition and development of products.
In addition, as more fully described below, Silo Pharma has entered into a license agreement with the University of Baltimore, Maryland, and has entered into a joint venture with Zylo Therapeutics, Inc., with respect to certain intellectual property and technology that may be used for targeted delivery of potential novel treatments. In addition, Silo Pharma has recently entered into a sponsored research agreement with Columbia University pursuant to which it has been granted an option to license certain patents and inventions relating to the treatment of Alzheimer’s disease and stress-induced affective disorders using Ketamine in combination with certain other compounds.
Silo Pharma plans to actively pursue the acquisition and/or development of intellectual property or technology rights to treat rare diseases, and to ultimately expand our business to focus on this new line of business.
Silo Pharma Signs Agreement to Develop First-in-Class Ketamine Implant Therapeutic
Initial indications are fibromyalgia and chronic pain
ENGLEWOOD CLIFFS, NJ, June 13, 2023 (GLOBE NEWSWIRE) — Silo Pharma, Inc. (Nasdaq: SILO) (“the Company”), a developmental stage biopharmaceutical company focused on merging traditional therapeutics with psychedelic research, today announced its entry into a research and development agreement to study and develop a dosage and time-release ketamine implant for the treatment of fibromyalgia. The research project includes analytical testing services and small batch pre-clinical proof of concept extrusion trials to determine drug release and stability.
“Alongside our development of SP-26, our novel time-release topical formulation of ketamine, we are beginning to explore an additional option for treating fibromyalgia using ketamine-loaded implants,” said Eric Weisblum, Chief Executive Officer of Silo Pharma. “The outcome of this research will provide additional information and data for our ongoing studies of ketamine treatments for fibromyalgia and other chronic pain indications.”
Fibromyalgia is a chronic condition causing widespread musculoskeletal pain accompanied by memory issues, sleep problems, and fatigue. The disorder affects about four million American adults, or about 2% of the adult population, and its treatment market is projected to grow at a compound annual growth rate of over 9% in the 2020-2027 period.1
SILO Pipeline:
.Silo Pharma believes in following the science to identify the best application for a therapeutic rather by targeting indications with the biggest need and selecting the best route of delivery for patients. Our current assets have shown promise in a range of illnesses and diseases, including Alzheimer’s; Parkinson’s; Multiple Sclerosis (MS); Rheumatoid Arthritis (RA); and Stress-Induced Psychiatric Disorders.
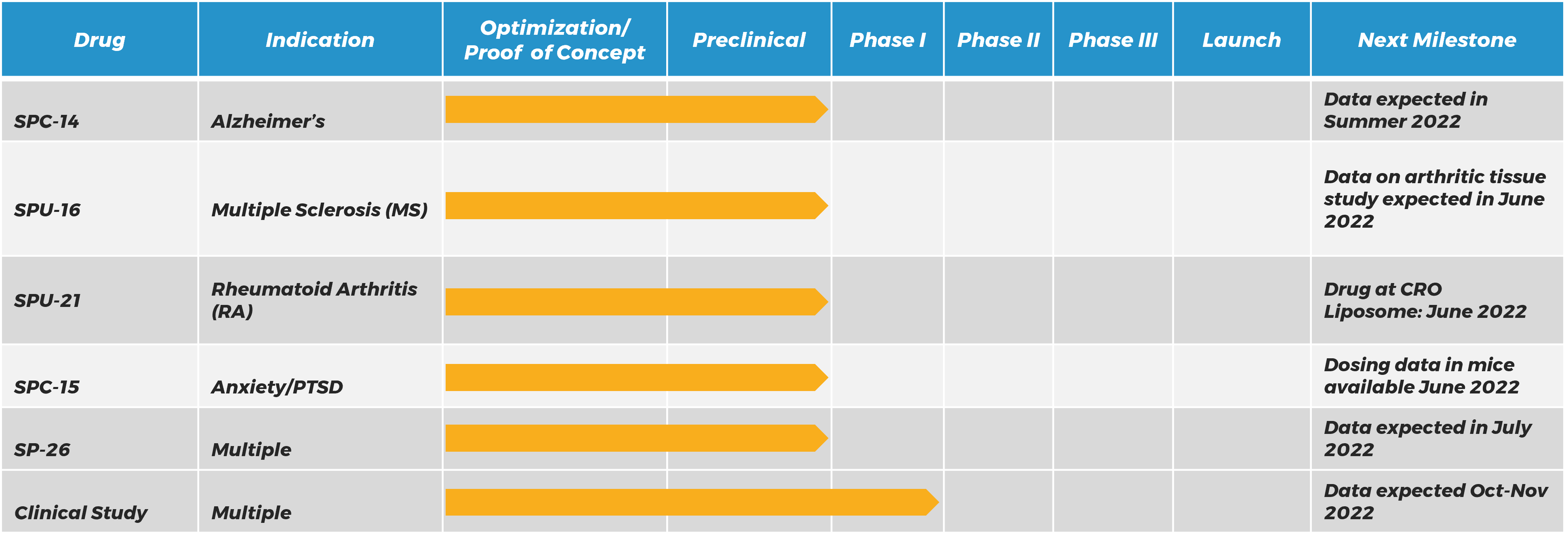
SILO Pharma’s Primary Asset Has Extraordinary Potential
505(b)(2) Pathway / preclinical testing and proof-of-concept being lead by inventor Dr. Christine Denny of Columbia University / SPC-14 has shown reduced hyponeophagia in animal studies / SPC-14 may reduce behavioral despair / Silo has licensed technology with Columbia and has recently entered into a scientific research agreement with Dr. Denny’s lab
Market Opportunity – Alzheimer’s Disease / 6.5 million Americans suffer from Alzheimer’s and related diseases / ~1 in 9 Americans 65+ have Alzheimer’s v U.S. market for relevant drugs expected to reach $5 billion by 2027
Technology – Licensed from Columbia University / SPC-14 is a novel drug combining two approved therapeutics / SPC-14 targets NDMARS and 5HT4Rs to treat cognitive and neuropsychiatric symptoms in Alzheimers.
 SPC-15 Targeted prophylactic treatment—Stress-induced affective disorders
SPC-15 Targeted prophylactic treatment—Stress-induced affective disorders
Sponsored Research Agreement with Columbia University Prevention of stress-induced affective disorders / Increasing stress resilience in military, first responders, and other populations at high risk of PTSD / Predicting the level of severity or progression such disorders / Molecular targets for use in drug discovery of innovative treatments.
Market Opportunity – Targeted prophylactic treatment—Stress-induced affective disorders / 26% of Americans 18+ suffer from anxiety, PTSD and other disorders / This number has escalated post-COVID-19 / U.S. market for relevant drugs expected to reach $13 billion by 2027
Technology – Metabolomic biomarkers predict response to pharmacological treatments / Utilizes ketamine compositions as a method for treatment and prevention.
 SP-26 Time-Released Psilocybin, Ketamine
SP-26 Time-Released Psilocybin, Ketamine
Deliver Ketamine or Psilocybin in a time-released manner / Will time-release diminish the hallucinogenic effects of these psychedelics / Pre clinical study underway shows Z-pod can hold and distribute Ketamine / Efficacy study in animals underway.
Market Opportunity – Time-Released Psilocybin, Ketamine / Multiple Indications
Technology – Joint Venture with Zylö Therapeutics, Inc. / Clinical development of psilocybin using ZTI’s Z-pod technology / Clinical development of Zylo’s sustained release topical delivery system.
May be used as a delivery tool to target current therapies to detect inflammation in the spinal cord / May be used for diagnosing and monitoring MS / Decreases toxicity of existing therapeutics / Animal study results show much improved delivery of therapeutics and decreased toxicity.
Market Opportunity – Multiple Sclerosis (MS) / There are approximately 400,000 Americans and 2.5 million people worldwide with MS / The most widespread disabling neurological condition of young adults / Global market for MS drugs expected to reach $25.3 billion by 202
Technology- Licensed from University of Maryland Baltimore / Patent issued / Central nervous system-homing peptides / Use for investigation and treatment of MS and other neuroinflammatory pathology.
 SPU-21 Arthritogenic Joint Homing Peptides Utilizing Psilocybin
SPU-21 Arthritogenic Joint Homing Peptides Utilizing Psilocybin
Identify markers of arthritic inflammation in joints / Isolate phage clones that preferentially target inflamed joints of arthritic Lewis rats / Peptide significantly inhibited arthritic progression in this animal model / Further studies are underway at UMB.
Market Opportunity – Rheumatoid Arthritis (RA) / 1.3M U.S. adults suffer from RA / The most common autoimmune disease in U.S. / U.S. market for RA drugs expected to reach $63 billion by 2027
Technology- Development plan to utilizing liposomal Homing Peptide to deliver targeted psilocybin / The ability of the peptides to target inflamed epithelium suggest they could be used to target drug delivery. This approach could enhance the therapeutic effect of current and future therapies and decrease potential systemic toxicity despite systemic administration of the drug. These peptides have potential for the development of fusion imaging molecules and/or nanoparticles to study arthritic pathogenesis. They could also be customizable and used to deliver nanoparticles for precise imaging. In addition, these novel joint-homing peptides can be used to treat autoimmune diseases, including but not limited to RA.
How SILO Pharma’s Drug Delivery Platform Could Cause Massive Changes to How Diseases Are Treated
Research has clearly shown that psychedelics – such as psilocybin – can offer significant benefits to those suffering from a number of disorders. But to date, the issue has been developing a psychedelic-based solution for these sufferers that was both practical and effective. Thanks to the potential of SILO Pharma’s unique drug delivery platform – developed in conjunction with university research – we could be on the verge of a significant breakthrough in treatment. SILO Pharma’s assets and research could be transformative to the well-being of patients worldwide…and disruptive to the healthcare industry as a whole. That alone sets Silo Pharma (OTC: SILO) apart from others in both the psychedelic and biopharma arenas.
The research and development – based on the company’s unique homing peptide – could not only prove to be effective in the delivery of psychedelics…but also in delivery of traditional medication. That type of potential is deserving of attention from investors…but it’s also more difficult to project. A potential comparable stock that has demonstrated the potential for this company’s upside, however, does exist in the cannabis space.
1) Double Play Investment Opportunity – Unlike many companies generating attention in the psychedelic space, SILO Pharma is focused on developing potential breakthroughs by combining traditional therapeutics with the power of psychedelic research.
2) Rapidly Growing Market – The market for psychedelics is projected to grow at a CAGR of 16.3% between now and 2027, when it is expected to reach $6.8 billion.
3) Potential Game-Changing Drug Delivery Platform – SILO Pharma’s potentially game-changing drug delivery platform may be used to treat a number of devastating diseases, including Rheumatoid Arthritis and Multiple Sclerosis and is likely to generate considerable attention from larger companies, making for attractive buyout potential.
4) Following a Proven Blueprint for Success – The company is following the same path forged in the cannabis sector by GW Pharma, and is working to combine the powerful healing benefits of psilocybin with its own unique drug delivery system in the hopes of developing a true game-changer that could ultimately prove disruptive to the entire healthcare industry.
5) Proven Leadership Team – Silo Pharma is led by an experienced team of business builders and researchers poised to make the company’s dream a reality. This team includes CEO Eric Weisblum, with over 20 years investing, building and managing businesses as well as an impressive scientific advisory board.
SILO RECENT NEWS
Silo Pharma Announces Participation on Webull Corporate Communications Service Platform
Silo Pharma Commences Formulation for SPC-15 Targeting Anxiety and PTSD Stress-Related Disorders
Silo Pharma Announces Expansion of Intellectual Property Portfolio
SILO EXECUTIVE TEAM
Silo Pharma’s is Led by an Experienced Management Team and an Impressive Group of Scientific Advisors
 CEO Eric Weisblum has over 20 years investing, building and managing businesses and will lead a scientific advisory board of reputable medical professionals to work and guide the company through the different stages of research, licensing, partnerships and trials.
CEO Eric Weisblum has over 20 years investing, building and managing businesses and will lead a scientific advisory board of reputable medical professionals to work and guide the company through the different stages of research, licensing, partnerships and trials.
Prior, Mr. Weisblum was President of Sableridge Capital for five years. Eric currently serves on the board of directors of Aikido Pharma., a Nasdaq listed biotech company focused on the commercialization of oncology therapeutics. In addition to being an active investor in both public and private companies, Mr. Weisblum has provided managerial assistance and guidance to help companies execute on their business strategy.
 Director Wayne Linsley has been an entrepreneur for over 40 years. In 1979, he received a Bachelor’s Degree in Business Administration from Sienna College in Loudonville, New York.
Director Wayne Linsley has been an entrepreneur for over 40 years. In 1979, he received a Bachelor’s Degree in Business Administration from Sienna College in Loudonville, New York.
He has since been involved with real estate brokerage and residential development, construction, finance, telecommunications. Since 2009 he has worked for and is currently a Vice President for CFO Oncall, Inc., a financial reporting firm that works with publicly traded companies.
 Director Kevin Munoz, MD, currently serves as Director of Operations at Physical Medicine and Rehabilitation where he has responsibility for the day-to-day management of all office operations with a focus on ensuring and increasing patient satisfaction. Prior to that, he led the configuration efforts during an enterprise-wide implementation of application software that also included streamlining and improving business processes.
Director Kevin Munoz, MD, currently serves as Director of Operations at Physical Medicine and Rehabilitation where he has responsibility for the day-to-day management of all office operations with a focus on ensuring and increasing patient satisfaction. Prior to that, he led the configuration efforts during an enterprise-wide implementation of application software that also included streamlining and improving business processes.
Sincerely,

DISCLAIMER
*****We have been compensated for this email.
THIS WEBSITE/NEWSLETTER IS A PUBLICATION OF ONE22 MEDIA, LLC, HEREIN REFERRED TO AS O22. O22’S REPORTS/RELEASES ARE A COMMERCIAL ADVERTISEMENT AND ARE FOR GENERAL INFORMATIONAL PURPOSES ONLY.O22 IS ENGAGED IN THE BUSINESS OF MARKETING AND ADVERTISING COMPANIES FOR MONETARY COMPENSATION. WE HAVE BEEN COMPENSATED A FEE OF EIGHTEEN THOUSAND USD FOR A ONE DAY SILO AWARENESS CAMPAIGN BY A THIRD PARTY, LEGENDS MEDIA, LLC.
WE HAVE BEEN COMPENSATED A FEE OF FIFTEEN THOUSAND FIVE HUNDRED USD FOR A ONE DAY SILO AWARENESS CAMPAIGN BY A THIRD PARTY, SICA MEDIA MEDIA, LLC. WE HAVE PREVIOUSLY BEEN COMPENSATED A FEE OF TWENTY FIVE THOUSAND USD FOR A ONE DAY SILO AWARENESS CAMPAIGN BY A THIRD PARTY, LEGENDS MEDIA, LLC.
BY SUBSCRIBING TO OR OTHERWISE USING THIS WEBSITE/NEWSLETTER, YOU AGREE TO HOLD O22 AND ITS OPERATORS, OWNERS, AND EMPLOYEES HARMLESS AND TO COMPLETELY RELEASE THEM FROM ANY AND ALL LIABILITY DUE TO ANY AND ALL LOSS, DAMAGE, OR INJURY THAT YOU MAY INCUR, MONETARY OR OTHERWISE.
INVESTING IN MICRO-CAP AND GROWTH SECURITIES IS HIGHLY SPECULATIVE AND CARRIES AN EXTREMELY HIGH DEGREE OF RISK. NEVER INVEST IN ANY STOCK FEATURED ON O22’S SITE OR NEWSLETTER UNLESS YOU CAN AFFORD TO LOSE YOUR ENTIRE INVESTMENT. THE DISCLAIMER IS TO BE READ AND FULLY UNDERSTOOD BEFORE USING O22’S SERVICES, JOINING O22’S SITE OR EMAIL/BLOG LIST, OR FOLLOWING ANY SOCIAL NETWORKING PLATFORMS O22 MAY USE.
PLEASE NOTE WELL: O22 IS NOT A REGISTERED INVESTMENT ADVISOR, BROKER DEALER OR A MEMBER OF ANY ASSOCIATION FOR OTHER RESEARCH PROVIDERS IN ANY JURISDICTION WHATSOEVER. O22 IS NOT AFFILIATED WITH ANY EXCHANGE, ELECTRONIC QUOTATION SYSTEM, THE SECURITIES AND EXCHANGE COMMISSION, OR FINRA. NONE OF THE MATERIALS OR ADVERTISEMENTS HEREIN CONSTITUTE OFFERS OR SOLICITATIONS TO PURCHASE OR SELL SECURITIES OF THE COMPANIES PROFILED.
THE INFORMATION CONTAINED HEREIN IS BASED ON INFORMATION SUPPLIED BY THE COMPANIES PROFILED, PUBLICLY AVAILABLE INFORMATION, PRESS RELEASES, AND OTHER SOURCES WHICH O22 BELIEVES TO BE RELIABLE, BUT IS NOT GUARANTEED BY O22 AS BEING ACCURATE AND DOES NOT PURPORT TO BE A COMPLETE STATEMENT OR SUMMARY OF THE AVAILABLE DATA. O22 IS NOT RESPONSIBLE FOR ANY CLAIMS MADE BY THE COMPANIES PROFILED. INVESTORS SHOULD NOT RELY ON THE INFORMATION CONTAINED IN THIS WEBSITE/NEWSLETTER IN DECIDING TO INVEST OR MAKE OTHER FINANCIAL DECISIONS. RATHER, INVESTORS SHOULD USE THE INFORMATION CONTAINED IN THIS WEBSITE/NEWSLETTER AS A STARTING POINT FOR DOING ADDITIONAL INDEPENDENT RESEARCH ON THE FEATURED COMPANIES. O22 STRONGLY ENCOURAGES READERS AND INVESTORS TO CONDUCT A COMPLETE AND INDEPENDENT INVESTIGATION OF THE RESPECTIVE COMPANIES, INCLUDING BY REVIEWING SEC FILINGS (FORMS 10-Q, 10-K, 8-K, 3, 4, 5, SCHEDULE 13D) AND BY CONSULTING YOUR OWN TAX, BUSINESS, FINANCIAL, AND INVESTMENT ADVISORS.
THE PRIVATE SECURITIES LITIGATION REFORM ACT OF 1995 PROVIDES A SAFE HARBOR IN REGARD TO FORWARD-LOOKING STATEMENTS. ANY STATEMENTS THAT EXPRESS OR INVOLVE DISCUSSIONS WITH RESPECT TO PREDICTIONS, EXPECTATIONS, BELIEFS, PLANS, PROJECTIONS, OBJECTIVES, GOALS, ASSUMPTIONS OR FUTURE EVENTS OR PERFORMANCE ARE NOT STATEMENTS OF HISTORICAL FACT, AND MAY BE FORWARD-LOOKING STATEMENTS. FORWARD-LOOKING STATEMENTS ARE BASED ON EXPECTATIONS, ESTIMATES, AND PROJECTIONS AT THE TIME THE STATEMENTS ARE MADE THAT INVOLVE A NUMBER OF RISKS AND UNCERTAINTIES WHICH COULD CAUSE ACTUAL RESULTS OR EVENTS TO DIFFER MATERIALLY FROM THOSE PRESENTLY ANTICIPATED. FORWARD-LOOKING STATEMENTS MAY BE IDENTIFIED THROUGH USE OF WORDS SUCH AS PROJECTS, FORESEES, EXPECTS, ANTICIPATES, ESTIMATES, BELIEVES, UNDERSTANDS, MAY, COULD, OR MIGHT. THERE IS NO GUARANTEE THAT PAST PERFORMANCE WILL BE INDICATIVE OF FUTURE RESULTS.

Recent Posts

Clean Vision Corp (OTC: CLNV): Overcoming the Plastic Waste Crisis

Meta Materials (NASDAQ: MMAT): More Due Diligence and Exploring Latest Developments

Integrated Cannabis Solutions’ (OTC: IGPK) 633% Surge: Exploring Catalysts, Company Overview, and Growth Potential in 2024

Sonoma Pharmaceuticals (NASDAQ: SNOA): Potential Surge to Speculations – What Lies Ahead?

1847 Holdings (NYSE: EFSH) Soars: Insights, Acquisitions, and What Lies Ahead




