Energy & Resources
Comeback Time for Delcath Systems, Inc. (OTCMKTS: DCTH)
Published
6 years agoon
Delcath Systems, Inc. (OTCMKTS: DCTH) has been seeing increased action since reversing off $0.23 lows. Last September was an epic month for DCTH; from a start point of around $2 DCTH ran to highs over $1o per share. DCTH used to be a NASDAQ runner but was delisted last year after the Company’s board failed to approve a reverse stock split. DCTH began trading on the OTCBB on Friday, September 15.
On December 26 Delcath Systems announced the company has entered into a definitive licensing agreement for CHEMOSAT® commercialization in Europe with medac Gesellschaft für klinische Spezialpräparate mbH (medac), a privately held, multi-national pharmaceutical company based in Hamburg area, Germany. Founded in 1970, medac specializes in the treatment and diagnosis of oncological, urological and autoimmune diseases. The company has offices globally, worldwide partner agreements in over 90 countries, and approximately 1,200 employees. Under the terms of the seven-year agreement, Delcath’s European subsidiary, Delcath Systems, Ltd. exclusively licenses medac to sell and market CHEMOSAT in all member states of the European Union, Norway, Liechtenstein, Switzerland, and the United Kingdom. medac will pay Delcath €6,000,000 in a combination of upfront and success-based milestone payments. Additionally, Delcath will receive a fixed transfer price per unit of CHEMOSAT as well as royalties. The agreement has a projected value of up to $45 million over the first seven-year term and includes an optional five-year extension.
Delcath Systems, Inc. (OTCMKTS: DCTH) is an interventional oncology Company focused on the treatment of primary and metastatic liver cancers. DCTH investigational product – Melphalan Hydrochloride for Injection for use with the Delcath Hepatic Delivery System (Melphalan/HDS) – is designed to administer high-dose chemotherapy to the liver while controlling systemic exposure and associated side effects.
The Company has commenced a global registration trial (The FOCUS Trial) for Patients with Hepatic Dominant Ocular Melanoma (OM) and have initiated a global Phase 3 trial (The ALIGN Trial) for patients with intrahepatic cholangiocarcinoma (ICC). Melphalan/HDS has not been approved by the U.S. Food & Drug Administration (FDA) for sale in the U.S.
In Europe, Melphalan/HDS has been commercially available since 2012 under the trade name Delcath Hepatic CHEMOSAT® Delivery System for Melphalan (CHEMOSAT), where it has been used at major medical centers to treat a wide range of cancers of the liver.
Last year DCTH initiated their registration trial of Melphalan Hydrochloride for Injection for use with the Delcath Hepatic Delivery System (Melphalan/HDS) to treat patients with intrahepatic cholangiocarcinoma (ICC). Called The ALIGN Trial, this trial will seek to enroll approximately 295 ICC patients at approximately 40 clinical sites in the U.S. and Europe. The trial is being conducted under a Special Protocol Assessment (SPA) agreement reached with the U.S. Food and Drug Administration (FDA) in March 2017. The ALIGN Trial is based on a strong efficacy signal observed in the ICC tumor type through our commercial experience with CHEMOSAT in Europe. We are leveraging our existing network of trial sites from our FOCUS Phase 3 trial to rollout the trial protocol as efficiently as possible and have 3 centers open for patient enrollment to date. In this orphan population where there exists a huge unmet need, this trial provides us with a second pathway to commercial drug approval in the United States, and if successful we believe will be an important value driver for the Company.
Last month DCTH announced the first patient has been enrolled under the amended protocol for its registration trial in ocular melanoma liver metastases. Stanford University Medical Center is the first trial site to enroll a patient under the amended protocol.
To Find out the inside Scoop on DCTH Subscribe to Microcapdaily.com Right Now by entering your Email in the box below
The trial, A Single-arm, Multi-Center, Open-Label Study to Evaluate the Efficacy, Safety and Pharmacokinetics of Melphalan/HDS Treatment in Patients with Hepatic-Dominant Ocular Melanoma (The FOCUS Trial), will enroll a minimum of 80 patients with ocular melanoma metastatic to the liver. Patients previously enrolled in the Melphalan/HDS arm of the trial under the prior randomized protocol will continue to be treated and evaluated as part of the amended trial.
On the licensing agreement for CHEMOSAT CEO said Jennifer K. Simpson, Ph.D., MSN, CRNP stated:
“With offices throughout Europe and a well-established network among oncology key opinion leaders, medac is a well-suited partner to help advance CHEMOSAT commercialization in the European Union and neighboring countries. Medac provides the organizational scale, expertise and market reach necessary to help us strive to firmly establish CHEMOSAT in the European treatment landscape for cancers of the liver. Additionally, through this agreement, we obtain immediate resources to support our efforts to advance our clinical development program. This is a highly significant development for Delcath, and we are pleased to be working with a market leader like medac to help this therapy fully realize its potential.”
We have a Monster Pick Coming. Subscribe Right Now!
Currently trading at a $2.8 million market valuation DCTH has $1.2 million in the treasury and $4.2 million in current assets and carries a very significant debt load of $18 million in current liabilities which has unfortunately put the power in the hands of the note holders. DCTH has small but growing revenues reporting $858,000 in sales for the 3 months ended June 30, 2018 up from $584,000 for the same period last year. We will be updating on DCTH when more details emerge so make sure you are subscribed to Microcapdaily so you know what’s going on with DCTH.
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Disclosure: we hold no position in DCTH either long or short and we have not been compensated for this article.
You may like
Energy & Resources
Buyer Group International’s (OTC: BYRG) Journey: Unearthing Potential with Strategic Exploration
Published
7 months agoon
December 14, 2023

Background:
BYRG is a junior exploration company aiming to revitalize the once-dormant “New Rambler District” nestled in Albany County, Wyoming. At the heart of this district lies the company’s flagship asset—the Shambhala project. The Shambhala project spans 1,466 acres and consists of 84 lode claims, granting BYRG exclusive rights to explore, mine, and extract minerals within this extensive property. The project is well known for the high grade intercepts of Platinum Group Metals (PGMs) which appears to have been exclusively extracted from this district in the early 1900s.
What’s interesting is the strategic location of the New Rambler District, positioned in close proximity to the southern boundary of the Cheyenne Belt—a renowned Greenstone Belt within the Medicine Bow mountains.
Greenstone Belts have an intriguing association with a diverse range of valuable minerals and precious metals, rendering them alluring targets for exploration and mining ventures.
What adds further fascination is the presence of two complexes in the central and east-central regions of the Medicine Bow Mountains. These complexes resemble both the Bushveld Complex in South Africa and the Stillwater Complex in Montana, renowned for containing the world’s primary reserves of platinum, palladium, osmium, ruthenium, iridium, and rhodium. Not to mention noteworthy quantities of chromium, vanadium, and gold.
According to BYRG, the geological complex found in the Medicine Bow Mountains is one of only three known in the world. The other two complexes similar to this are highly valued and have been proven to be worth tens of billions. Given these remarkable geological similarities, there is a strong possibility that the Medicine Bow Mountains may contain valuable mineral resources, including critical and rare strategic metals, as well as highly significant precious metals.
History & Geology:
The New Rambler mine’s history dates back over 120 years, with exploration beginning in the year 1900. BYRG has shared all kinds of historic write-ups from the newspaper to prove this, it’s quite fascinating.
What matters is BYRG’s mineral deposit that’s formed near the surface. The company has shared an in depth geological explanation of it on their website, but we’ve simplified it for the average investor below.
Their deposit hosts a layer of rusty, porous material (limonite and jaspilite gossan) covering a zone about 75 feet thick that was changed by oxygen. In this zone, there were copper minerals in the form of carbonates and oxides. These included some copper that looked like tree branches (dendrites) and chunks of pure copper, along with a bit of copper mixed with sulfur.
As you went deeper, this zone changed into another mineral layer with covellite and chalcocite minerals that carry platinum. Further down, past 100 feet, there were different rocks with minerals like quartz, pyrite, and chalcopyrite. The combination of platinum and palladium found together in certain zones suggests that the valuable ore might have moved from a hidden source deep underground, not yet discovered, or even laterally.
The mine operated off and on between 1900 and 1918 but stopped when a fire destroyed the mine buildings. Active mining never resumed at this site, and as a result very little information on these mines exists in geological literature. Nearly all of the old mine buildings, mine maps, reports, and records were lost to the fire.
Subscribe to Microcapdaily.com Right Now by entering your Email in the box below.
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
2022 & 2023 XRF Readings:
Before we get into the readings, it’s important to understand what XRF readings are. They’re commonly used across various fields including geology, mining, environmental science, archaeology, material science, and more for determining the elemental composition of substances without damaging the sample.
More specifically, an XRF reading displays the quantitative analysis of the elements detected in the sample. It provides information about the types and concentrations of elements present in the material being analyzed. This data is often presented as a spectrum or a list of elements along with their respective concentrations or percentages.
2022:
In the exploration season of 2022, BYRG acquired substantial XRF readings from their Shambhala #71 adit, outcroppings, and tailings. This uncovered noteworthy discoveries. Surprisingly, they identified multiple high-grade intercepts containing various metals such as cobalt, zinc, palladium, platinum, and rhodium. For specific examples of these readings, please review this link.
The results saw unexpected high grade Cobalt, a key critical mineral used in electric vehicles. On top of this, bonanza palladium intercepts, reaching an impressive 93 grams per ton, where 6 grams per ton is typically classified as “high grade”.
Additionally, bonanza platinum intercepts registered as high as 40 grams per ton, surpassing the 6 grams per ton threshold considered “high grade”.
Furthermore, multiple high-grade intercepts of rhodium, valued as the world’s most precious metal at $14,000 USD per ounce, were also identified.
2023:
The 2023 report delves into the Joker mine region, situated south of both the Shambalah and New District mines, encompassing key elements like the shaft house, cabins, mine, and tailings.
Once again, the XRF readings revealed notable high-grade intercepts across a diverse spectrum of metals. Impressively, these readings covered an array of elements including Aluminum (Al), Titanium (Ti), Iron (Fe), Cobalt (Co), Palladium (Pd), Vanadium (V), Copper (Cu), Nickel (Ni), Yttrium (Y), Manganese (Mn), Rhodium (Rh), Tantalum (Ta), and Niobium (Nb).
Highlighted in bold on the release were high-grade results for Aluminum, Titanium, Iron, Cobalt, Palladium, Vanadium, and Rhodium standing out among the listed metals.
Dave Bryant, CEO of Buyer Group, expressed satisfaction, stating, “We are extremely pleased to witness the consistent continuation of high-grade findings at the Joker Mine, particularly in proximity to Shambhala #71 adit last year.” He highlighted successful interceptions of high-grade rhodium, palladium, cobalt, and various other minerals crucial to the United States’ domestic supply chain across multiple tests. Bryant added, “Given the scale of Project Shambhala, the potential continuity of this mineralized zone at depth could yield a substantial supply of strategic metals.”
Latest Update:
BYRG released an update on October 19, 2023, regarding their progress in the exploration process. Here’s a brief summary of the announcement.
BYRG is excited to present a technical memorandum summarizing Hard Rock Consulting’s (HRC) surface sampling activities conducted during the summer of 2023 within’ the Shambhala claims area.
This memorandum also delivers a detailed and precise overview of the geological history and landscape of the region. Following the acquisition of assay results, HRC intends to continue expanding these efforts, aiming towards Phase 2 exploratory activities and drilling.
“As we approach the conclusion of Phase 1 within our sequential phases leading to the development of a mineral resource estimate for the Shambhala Project,” stated Dave Bryant, CEO, “HRC will initiate the compilation of the final Phase 1 report upon receipt of laboratory results from AAL. This comprehensive study will serve as the groundwork for our subsequent strategies, leading us directly into Phase 2, involving the preparation of an NI 43-101 technical report. This report will encompass the project’s conceptual geological model and offer recommendations for future exploration through drilling initiatives.”
Share Consolidation:
Announced this August, $BYRG effectively retrieved an additional 192,808,718 shares from Alpine/Scottsdale, transferring them back to the company’s transfer agent and out of problematic hands. Efforts are underway to reclaim a 33 million-share block, leaving just one block with the broker, Alpine. Post the retrieval of Alpine’s share blocks, the focus will shift towards addressing the persisting BNY Mellon block comprising over 2 billion shares.
For investors, this series of share retrievals represents a positive move toward streamlining and securing the company’s share structure. By removing shares from potentially disruptive hands and consolidating control, it fosters improved stability and better control over the company’s equity. This action suggests an initiative to fortify investor confidence by mitigating potential dilution risks and establishing a more solid foundation for future growth and shareholder value.
Subscribe to Microcapdaily.com Right Now by entering your Email in the box below.
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
What Happened & What’s Next:
The excitement among online investors seems to revolve around the expectations for the 2023 exploration and assay results. For those not acquainted with the procedure, during drilling, exploration companies retrieve core samples that need testing in a laboratory to determine the metal concentrations in the ground. The duration for this process varies, contingent upon several factors, ranging from a few weeks to several months.
Very recently, BYRG shared on Twitter their invitation from American Assay Laboratories to visit their facilities. They mentioned that this is the same company responsible for recently completing their laboratory results, which are expected to be released very shortly.
Should there be further noteworthy findings and additional high-grade intercepts, this could serve as a significant catalyst, changing BYRG’s trajectory. We recommend investors maintain a close watch as this development will hold considerable importance.
Conclusion:
To clarify, investing in BYRG involves considerable risk, and extensive exploration efforts lie ahead. However, considering the underlying geological foundation, recent XRF readings, and the historical viability of an economical mine in the early 1900s, there’s potential for rapid and intriguing developments.
An important point to consider is that currently, only one company in the entire United States is extracting PGMs. Statistical evidence indicates production challenges, with the lowest levels seen since 2017 and notably low reserves at 900,000 oz. There’s a need for a player to step forward in this sector, could it be BYRG? There’s certainly a possibility, although more certainty can only come after a full NI 43-101 technical report, which BYRG aims to achieve by 2025-2026.
We will update you on BYRG when more details emerge, subscribe to Microcapdaily to follow along!
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Disclosure: We have not been compensated for this article/video. MicroCap Daily is not an investment advisor; this article/video does not provide investment advice. Always do your research, make your own investment decisions, or consult with your nearest financial advisor. This article/video is not a solicitation or recommendation to buy, sell, or hold securities. This article/video is our opinion, is meant for informational and educational purposes only, and does not provide investment advice. Past performance is not indicative of future performance.
Energy & Resources
iSun, Inc (NASDAQ: ISUN) on the Rise: Recent Market Growth & Remarkable Momentum
Published
8 months agoon
November 29, 2023
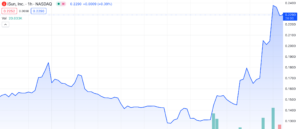
iSun, Inc (NASDAQ: ISUN) has experienced a significant surge of over 88% since November 22nd, 2023, with 12% of that increase observed within the current trading day. Similar to other Nasdaq-listed companies facing minimum bid requirement challenges, iSun has witnessed this remarkable upswing without any corresponding press releases or SEC filings to justify the sudden uptick. Looking further, we aim to uncover the underlying factors propelling this sudden rise by examining the company’s recent material events and financial performance, providing a comprehensive evaluation of what the future might hold for ISUN.
Background:
ISUN’s been in business since 1972, so quite a long while. After 47 years of being private, they decided to go public in June, 2019 and were previously known as The Peck Company.
Following the acquisition of ISUN, the company underwent a name change, becoming the entity known today. They have since taken the lead in integrating influential electrification technologies.
With a longstanding reputation as a reliable service partner for Fortune 500 firms, ISUN has a track record of installing various technologies, including clean rooms, fiber optic cables, flight simulators, and over 600 megawatts of solar systems.
The company offers an extensive array of solar services covering residential, commercial, industrial & municipal, as well as utility-scale projects.
Highlighting the importance of shifting towards clean, renewable solar energy, it’s worth noting that ISUN is committed to seizing profitable growth opportunities by offering solar electric vehicle charging solutions for both grid-tied and battery-backed systems.
Latest Earnings:
Unlike the last few write ups, we’re fortunate to have access to more informative press releases and SEC filings for ISUN. That said, let’s talk about their latest earnings and how the business is currently performing.
Highlights by numbers:
- Q3 2023 revenue of $27.9 million, up 47% from Q322, as continued strong commercial and industrial execution drives growth
- YTD revenue of $70.3 million, up 39% over first nine months of 2022
- Gross profit of $5.4 million, up 50% from Q322
- Gross margin of 19.45%, up 45 basis points from 19.0% in 2022’s third quarter, as benefits from synergies were offset by mix
- Awarded $27.0 million in new solar and EV infrastructure contracts in Q3 2023, with a total of $67.0 million in first nine months of 2023
- Continuing successful execution of growth strategy, leveraging tailwinds
Okay great, both the revenue and profit margins are moving in a positive direction. In this market, let’s be honest, what truly matters is whether this long-standing company can generate profits while tackling the substantial growth potential in renewable solar energy projects.
There’s a few things worth noting from this release, the bigger point being the heavy tailwinds pushing them into record growth this year. Their commercial and industrial division showed impressive growth, achieving a 47% revenue boost and a 45 basis point increase in margins compared to the previous year. Despite challenges due to higher interest rates for residential customers, the team remains focused on seizing opportunities driven by climate policies and growing interest among customers in alternative energy solutions.
On another front, their operating loss in Q323 was ($1.8) million, a $3 million or 64% improvement compared to a loss of ($4.9) million in Q322. The cause for this? Higher revenue and lower operating expenses.
To be frank, the majority of CEOs across various industries anticipate higher productivity with fewer resources, which can be a continual source of high stress for employees actually accomplishing the daunting dask. Nethertheless, ISUN is achieving record growth with ease…
As a final thought on operating income, their YTD loss was ($6.2) million, a $10 million or 62% reduction compared to a loss of ($16.2) million during the same period in 2022.
Subscribe to Microcapdaily.com Right Now by entering your Email in the box below.
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Thoughts from the CEO:
“We continue to make substantial progress towards the targets we set for iSun’s performance this year, as we execute on our strategy and fulfill our commitments to investors. We remain confident that our expanded capabilities effectively address the needs of more customers and position us to accelerate our growth in the evolving alternative energy sector. Our continued success in winning significant contracts with existing and new customers reflects the appeal of our platform approach that delivers a suite of services to meet the needs of diverse customers. This year, we are also benefiting from the expertise of our team in executing efficiently on our backlog to address our customers’ needs and leveraging the relationships and partnerships we have established. Now that our country’s energy policy has been established for the next 10 years through the IRA legislation passed in 2022, we expect those factors to help us scale our operations significantly in the next few years, no matter what macroeconomic challenges may persist, and thus enable us to generate steadily higher revenue and reach operating profitability in the years ahead.”
Thoughts from Retail:
Several types of traders on Twitter are actively discussing ISUN, many with tens of thousands of followers. One in particular with a 72.1K following on Twitter, @MoonMarket_, mentions he’s accumulating for potentially larger moves ahead, based on technical trends.
@greatstockpicks and @FrankieBstock, with a combined following of 58.4K, are also actively discussing ISUN. Their sentiments lean positively, indicating that the deeper they dive into the company, the more appealing it appears.
Frankie also highlights, “Wainwright has a .50 target…given the revenue, growth, low offering risk and 161m backlog I could see it going higher but let’s focus on .50 with a stop loss around .12”.
It’s certainly encouraging to witness higher attention from influential sources discussing a stock, but it’s always crucial to conduct your own research and form an independent judgment.
Ultimately, all we can do is hope for the best regarding anyone’s analysis, even reputable analysts. While they are probably more reliable than figures like Cramer, who is often criticized for his inaccuracies concerning small-cap stocks, it’s essential not to take opinions as absolute truths.
Analyst Coverage:
As mentioned, analysts might not always be the most accurate to follow, but it’s usually reassuring when individuals with robust financial backgrounds, widespread industry connections, and expertise provide their insights on a public company.
ISUN is presently under analysis by three different firms: Alliance Global, Roth Capital Partner, and H.C. Wainwright & Co. They have set varying buy recommendations and price targets for the company, with a low, median, and high range of $0.50, $2.00, and $2.75, respectively. That’s a monumental 127.4%, 809.9%, and 1151.1% increase should ISUN meet those expectations.
Subscribe to Microcapdaily.com Right Now by entering your Email in the box below.
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
What’s Next:
ISUN seems to primarily focus its market updates on financial aspects. Upon reviewing all their 2023 releases, they consistently highlight robust execution and growth, reinforcing the anticipation of total revenue reaching $95-100 million this year, indicating a 24-31% surge compared to 2022.
As mentioned earlier, ISUN currently operates at a loss, and often experiences fluctuating quarters due to the impact of high-value contracts. These contracts can significantly influence the company’s financial results.
Thankfully, ISUN holds a total backlog of $161.8 million as of September 30, 2023, ensuring a stable revenue stream as projects are finalized. The bulk of this backlog, approximately $140.3 million, pertains to commercial and industrial applications, projected to be finished within a span of 10-18 months.
As is common with non-profitable businesses, it’s crucial to stay watchful for potential near-term dilution. Fortunately, ISUN recently secured term sheets for a non-dilutive $8 term loan, providing a reassuring stance for the time being. Should the management maintain this level of execution, achieving profitability may not be far off and would be a substantial milestone.
Regarding Nasdaq’s minimum bid requirement of $1.00, ISUN received an extension until May 2024, aiming to facilitate an organic increase in their stock price without the risk of a reverse split.
Conclusion:
With a long-standing history and a shift towards electrification technologies, ISUN’s dedication to renewable solar energy and diverse services positions it for substantial growth.
The overarching market trend aligns favourably, offering support to their endeavours, while the management team’s restructuring on cost efficiencies bode well for near term profitability. Their recent earnings demonstrated substantial revenue growth, particularly in their commercial and industrial segments where they’ve overcome significant market challenges.
In the realm of undervalued micro-cap stocks listed on the NASDAQ, ISUN emerges as a compelling candidate worth monitoring closely.
We will update you on ISUN when more details emerge, subscribe to Microcapdaily to follow along!
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Disclosure: We have not been compensated for this article/video. MicroCap Daily is not an investment advisor; this article/video does not provide investment advice. Always do your research, make your own investment decisions, or consult with your nearest financial advisor. This article/video is not a solicitation or recommendation to buy, sell, or hold securities. This article/video is our opinion, is meant for informational and educational purposes only, and does not provide investment advice. Past performance is not indicative of future performance.
Picture by andreas160578 from Pixabay.com
Energy & Resources
BlueFire Technology Corp. (OTC: BLFR): Bold Ambitions & Path to NASDAQ Uplisting
Published
9 months agoon
November 3, 2023
Over the past month, BlueFire Equipment Corporation (OTC: BLFR) has witnessed an astounding surge of 1900%, soaring from its recent low of approximately $0.021 to a peak of $0.42. While it has recently stabilized at around the $0.34 mark, several noteworthy announcements have emerged, capturing the attention of online investors. Nevertheless, it remains somewhat undiscovered in the broader market landscape. With the potential for a NASDAQ up listing, can this relatively obscure OTCPNK company break into the major leagues? To gain a better understanding of their prospects, let’s delve deeper into what BLFR is all about.
Background:
BLFR seemed to function more or less as a shell company until a recent shift in its operations. Despite a flurry of press releases, BLFR has not yet developed a website with an IR section to review resources. The company is clearly in very early stages, yet it’s already surged 1900%. That alone renders this speculative investment particularly intriguing, considering there’s many more high-value milestones ahead.
In the realm of OTC-traded companies, no website isn’t entirely unusual. It’s usually possible to uncover a wealth of information by sifting through press releases and SEC filings.
After some digging on our end, it appears their focus is in the energy sector, where they’ve now just acquired 90% of a cash flow positive family-owned oil & gas company in the state of Texas. Let’s delve into the transaction to better understand the company and what lays ahead.
Subscribe to Microcapdaily.com Right Now by entering your Email in the box below.
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
The Transaction:
In an all-stock transaction, Screaming Eagle was merged into BLFR, and in exchange, the owners received 45,000,000 shares of Preferred Stock Series A and 810,000 shares of Preferred Stock Series B. Matthew Goldston, the current CFO of Screaming Eagle, has taken on the role of CFO at BLFR, while Nickolas S. Tabraue continues as a Director of the Board, Interim CEO, CCO, and CIR. Additionally, Kirk Yariger has been appointed as the Chairman of the Board, and Jonas Crafts joins as a Director of the Board at BLFR.
Screaming Eagle’s Short-Term Plan:
- Starting in Q4 2023, Screaming Eagle will launch a three-well horizontal side track drilling program on the Bedias Creek asset and an eleven-well clean-out program on the Gin Creek Asset in collaboration with 50% operating partner Exponent.
- Screaming Eagle has identified over 200 drill sites on this property and has identified more than 5 stacked pay zones.
- Current wells were drilled on 700 acres spacing with only 40 acres being depleted in each well.
- The plan involves drilling horizontal slim hole sidetracks in existing vertical well bores with flat decline curves in producing zones.
- The expected initial production of the first 6 wells is 800-1,200 bbls/day.
- Screaming Eagle anticipates acquiring an additional 1,800 bbls/day in Q4 2023 through the acquisition of another blue-chip producing asset in Texas with shale and Austin Chalk producing formations.
BLFR’s Short-Term Plan:
- BLFR plans to engage a firm to assist in working towards uplisting to the Nasdaq.
- They will engage a PCAOB auditor to commence a company audit.
- BLFR intends to cancel 18 million shares of the Company’s Outstanding Common Stock.
- The company plans to change its name and stock symbol to better align with its new direction.
- BLFR aims to create an informative website for investors.
- After completing the stock symbol and name change, they will engage a branding and marketing firm for their new direction.
Subscribe to Microcapdaily.com Right Now by entering your Email in the box below.
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Significant Twitter Updates:
BLFR Reserve Study:
A glance at BLFR’s Twitter feed displays their reserve study report where assets are currently valued at USD $96,556,640. Their current market capitalization stands at approximately $10M which seems strikingly low considering the well report. But brace yourself for the next piece of information they shared – it suggests the potential for a staggering $142,341,150 in future net income too.
Shareholder Friendly:
If we look further into their AS (Authorized Shares) and OS (Outstanding Shares) it’s fairly high with 2B AS. It’s worth noting that BLFR just recently planned on reductions given the company’s latest comments on Twitter, “there is no need to have more than 250,000,000 shares authorized to manifest our vision while maintaining share integrity and increasing value.”
Reducing the authorized share count is a proactive step by management, significantly diminishing the potential for future dilution, which undoubtedly benefits shareholders.
NASDAQ Minimum Bid:
The NASDAQ requires a minimum bid of $5.00 for any company going through the uplist process. As you know, BLFR is currently working with Eventus Advisory Group to uplist to the NASDAQ.
What’s very interesting is a tweet the company put out just recently, “$BLFR’s management is confident that there will not be a need for a reverse split to meet NASDAQ’s $5.00 minimum bid requirement.”
For those that aren’t familiar a reverse split is a corporate action where a company reduces the total number of its outstanding shares by consolidating them into a smaller number of shares. In essence, it’s the opposite of a regular stock split.
This would naturally result in each individual share having a higher numerical value. However, it’s crucial to keep in mind that with fewer shares outstanding, the increased per-share value doesn’t translate into an overall increase in the value of your investment, it’d still be worth the exact same amount.
The essence of this tweet is that the company has strong confidence in its intrinsic value, and upcoming catalysts could potentially propel the company to trade at a significantly higher valuation. The current stock price is $0.33 at time of writing, achieving $5.00 per share would represent a remarkable gain of over 1400%, making this a bold and ambitious assertion by the management.
Conclusion:
Considering the early stage of this investment and the rapidly evolving landscape, we anticipate numerous developments unfolding shortly. We’re committed to providing regular updates as the company progresses with its vision.
It’s clear there are some fundamental tasks on the agenda, like setting up a website and undergoing a name change, but we can’t help but be optimistic about the array of promising opportunities that seem to be just around the corner, potentially yielding massive returns for investors.
We will update you on BLFR when more details emerge, subscribe to Microcapdaily to follow along!
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Disclosure: We have not been compensated for this article/video. MicroCap Daily is not an investment advisor; this article/video does not provide investment advice. Always do your research, make your own investment decisions, or consult with your nearest financial advisor. This article/video is not a solicitation or recommendation to buy, sell, or hold securities. This article/video is our opinion, is meant for informational and educational purposes only, and does not provide investment advice. Past performance is not indicative of future performance.
Recent Posts

Clean Vision Corp (OTC: CLNV): Overcoming the Plastic Waste Crisis

Meta Materials (NASDAQ: MMAT): More Due Diligence and Exploring Latest Developments

Integrated Cannabis Solutions’ (OTC: IGPK) 633% Surge: Exploring Catalysts, Company Overview, and Growth Potential in 2024

Sonoma Pharmaceuticals (NASDAQ: SNOA): Potential Surge to Speculations – What Lies Ahead?

1847 Holdings (NYSE: EFSH) Soars: Insights, Acquisitions, and What Lies Ahead
Trending
-

 Uncategorized2 years ago
Uncategorized2 years agoMeta Materials Inc (OTCMKTS: MMTLP) Enormous Short Position in Trouble as Next Bridge Hydrocarbons Set to Stop Trading (George Palikaras & John Brda on Corporate Action)
-

 Micro Cap Insider3 years ago
Micro Cap Insider3 years agoMedium (KOK PLAY) The Parabolic Rise of Metal Arts (OTCMKTS: MTRT)
-

 Media & Technology3 years ago
Media & Technology3 years agoHealthier Choices Management Corp. (OTCMKTS: HCMC) Powerful Comeback Brewing as PMI Patent Infringement Lawsuit Moves Forward
-
Media & Technology4 years ago
AMECA Mining RM; the Rise of Southcorp Capital, Inc. (OTCMKTS: STHC)
-

 Media & Technology3 years ago
Media & Technology3 years agoSNPW (Sun Pacific Holding Corp) Power Brewing: 50MW solar farm project in Durango Mexico MOU with Atlas Medrecycler 48,000 SF New Partnership Queensland Australia Solar Farm.
-

 BioPharma2 years ago
BioPharma2 years agoAsia Broadband (OTCMKTS: AABB) On the Move Northbound Since Sub $0.08 Dip as Crypto Innovator Elevates AABB Crypto Exchange & Enters the NFT Space
-

 Uncategorized2 years ago
Uncategorized2 years agoMeta Materials Inc (OTCMKTS: MMTLP) Short Squeeze S-1a4 Filing Signals S1 Approval Could Be Days Away (Next Bridge Hydrocarbons Spin-Off)
-
 BioPharma3 years ago
BioPharma3 years agoHumbl Inc (OTCMKTS: HMBL) Major Reversal as Powerful Advisor Rejoins the Team & Looks to Uplist to Major Exchange