BioPharma
Anavex Life Sciences Corp (NASDAQ: AVXL) On Watch as Biotech to Present Results for its Phase 2b/3 Trial for blarcamesine for Alzheimer’s Disease
Published
2 years agoon
By
Boe Rimes
Anavex Life Sciences Corp (NASDAQ: AVXL) is an exciting biotech small cap caps targeting the holy grail of medicine; Alzheimer’s disease and other neurodegenerative diseases. The Company is at a pivotal point and will present results from its phase 2b/3 trial for blarcamesine for Alzheimer’s disease at the CTAD conference in San Francisco on December 1st; a condition of significant unmet need and economic burden for which there are only limited approved pharmacological treatment options. More on the phase 2b/3 trial in the conclusion of this article.
On November 7 AVXL announced the U.S. Food and Drug Administration’s (FDA) has granted Orphan Drug Designation (ODD) to ANAVEX®2-73 (blarcamesine) for the treatment of Fragile X syndrome. Fragile X syndrome (FXS) is the most common form of inherited intellectual disability and the most frequent single gene cause of autism spectrum disorder with an estimated population of approximately 62,500 in the US and 1,088,500 worldwide.1 At present, there is no approved treatment for Fragile X syndrome.
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
 Anavex Life Sciences Corp. (Nasdaq: AVXL) is a publicly traded biopharmaceutical company dedicated to the development of novel therapeutics for the treatment of neurodegenerative and neurodevelopmental disorders, including Alzheimer’s disease, Parkinson’s disease, Rett syndrome, and other central nervous system (CNS) diseases, pain, and various types of cancer.
Anavex Life Sciences Corp. (Nasdaq: AVXL) is a publicly traded biopharmaceutical company dedicated to the development of novel therapeutics for the treatment of neurodegenerative and neurodevelopmental disorders, including Alzheimer’s disease, Parkinson’s disease, Rett syndrome, and other central nervous system (CNS) diseases, pain, and various types of cancer.
Anavex’s lead drug candidate, ANAVEX®2-73 (blarcamesine), has successfully completed a Phase 2a clinical trial for Alzheimer’s disease, a Phase 2 proof-of-concept study in Parkinson’s disease dementia, and both a Phase 2 and a Phase 3 study in adult patients with Rett syndrome. ANAVEX®2-73 is an orally available drug candidate that restores cellular homeostasis by targeting sigma-1 and muscarinic receptors. Preclinical studies demonstrated its potential to halt and/or reverse the course of Alzheimer’s disease. ANAVEX®2-73 also exhibited anticonvulsant, anti-amnesic, neuroprotective, and anti-depressant properties in animal models, indicating its potential to treat additional CNS disorders, including epilepsy.
The Michael J. Fox Foundation for Parkinson’s Research previously awarded Anavex a research grant, which fully funded a preclinical study to develop ANAVEX®2-73 for the treatment of Parkinson’s disease. ANAVEX®3-71, which targets sigma-1 and M1 muscarinic receptors, is a promising clinical stage drug candidate demonstrating disease-modifying activity against the major hallmarks of Alzheimer’s disease in transgenic (3xTg-AD) mice, including cognitive deficits, amyloid, and tau pathologies. In preclinical trials, ANAVEX®3-71 has shown beneficial effects on mitochondrial dysfunction and neuroinflammation.
Microcapddaily first reported on AVXL on April 2, 2015 after AVXL had reversed off $0.15 lows on the OTCQB stating at the time: “Currently trading at a $12.8 million market valuation AVXL does have $7 million in the treasury and is fully funded going forward. Now that the stock has made a significant reversal off $0.15 after years of steady decline and the Company is presenting promising preclinical data for both ANAVEX 2-73 and ANAVEX 3-71, Investors are giving AVXL another look.
Upcoming Alzheimer's Disease Phase III results:@AnavexLifeSci's blarcamesine hydrochloride readouts expected this fall@Roche’s gantenerumab readout expected by the end of 2022@LillyPad’s donanemab-results expected in 2Q 2023@TrialsArena $AVXL $ROG $LLYhttps://t.co/faq49pCYWn
— Krzysztof Potempa (@BRAINCURES) November 8, 2022
To Find out the inside Scoop on AVXL Subscribe to Microcapdaily.com Right Now by entering your Email in the box below
On November 7 AVXL announced the U.S. Food and Drug Administration’s (FDA) has granted Orphan Drug Designation (ODD) to ANAVEX®2-73 (blarcamesine) for the treatment of Fragile X syndrome. Fragile X syndrome (FXS) is the most common form of inherited intellectual disability and the most frequent single gene cause of autism spectrum disorder with an estimated population of approximately 62,500 in the US and 1,088,500 worldwide.1 At present, there is no approved treatment for Fragile X syndrome.
The FDA’s Orphan Drug Designation program provides orphan status to drugs defined as those intended for the treatment, diagnosis or prevention of rare diseases that affect fewer than 200,000 people in the United States. Orphan drug designation qualifies the sponsor of the drug for certain development incentives, including tax credits for qualified clinical testing, prescription drug user fee exemptions and seven-year marketing exclusivity upon FDA approval. Anavex Life Sciences’ product portfolio platform includes drug lead candidate ANAVEX®2-73 for the treatment of Alzheimer’s disease, Parkinson’s disease dementia, Parkinson’s disease and Rett syndrome and ANAVEX®3-71 for schizophrenia, frontotemporal dementia, and Alzheimer’s disease.
AVXL CEO Christopher U Missling stated: “The Orphan Drug Designation highlights the potential to expand the therapeutic profile of ANAVEX®2-73 into the largest portion of autism spectrum disorder, Fragile X syndrome. We look forward to working with the Fragile X syndrome community to rapidly advance ANAVEX®2-73 as a potential treatment for Fragile X syndrome while we continue to expand late-stage clinical investigation of ANAVEX®2-73 as part of its precision medicine platform technology for both neurodevelopmental and neurodegenerative indications.”
$AVXL continue to dance sideways as the 30 and 10 weeks converge. This is incredibly healthy action for a sustained breakout pic.twitter.com/17RvejMt07
— Greg (@Dorman06) November 8, 2022
For More on AVXL Subscribe Right
AVXL is quickly emerging as one of the most exciting stocks in small caps targeting the holy grail of medicine; Alzheimer’s disease and other neurodegenerative diseases. Alzheimer’s is among the top 10 leading causes of deaths in the United States. As we have always said here at Microcapdaily on AVXL the Company has got everyone’s attention; AVXL is sitting on drugs in development that could potentially disrupt several billion-dollar markets. Anavex will present results from its phase 2b/3 trial for blarcamesine for Alzheimer’s disease at the CTAD conference in San Francisco on December 1st; a condition of significant unmet need and economic burden for which there are only limited approved pharmacological treatment options. In a past trial, Anavex’s blarcamesine modified mild Alzheimer’s disease in some patients at 148 weeks. At higher concentrations, 4 out of six individuals with mild Alzheimer’s disease or mild cognitive impairment improved at 57 weeks and 3 of these six individuals retained these improvements at 109 weeks. If these numbers hold up in the larger trial and blarcamesine meets safety requirements, the FDA could grant the drug accelerated approval. AVXL was a big runner that saw a historic rise starting in 2014 when we first covered it below $0.20 when it was trading on the OTCQB to over $30 per share on the NASDAQ during the summer of 2021. We will be updating on AVXL when more details emerge so make sure you are subscribed to Microcapdaily.
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
You may like
-


Anavex Life Sciences Corp (NASDAQ: AVXL) Running on Berenberg Coverage as ANAVEX2-73 (Blarcamesine) Takes on Alzheimer’s, Rett’s & Parkinson’s
-


Anavex Life Sciences Corp (NASDAQ: AVXL) ANAVEX2-73 (Blarcamesine) Gaining Fast – The Holy Grail of Alzheimer’s Disease (AD).
-


The Steady Rise of Anavex Life Sciences Corp. (Nasdaq: AVXL)
-
The Long and Short of Anavex Life Sciences Corp.(NASDAQ:AVXL)
-
Looking for a Cure: Anavex Life Sciences Corp.(NASDAQ:AVXL)
-
Anavex Life Sciences Corp.(NASDAQ:AVXL) Is Heating Up
BioPharma
Brainstorm Cell Therapeutics’ (NASDAQ: BCLI) Post-Setback Resurgence: Unpacking FDA Impact & Market Expectations
Published
8 months agoon
November 30, 2023
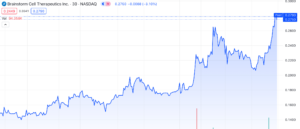
Brainstorm Cell Therapeutics (NASDAQ: BCLI) continues to gain momentum with a surge of over 125% since its low on October 31st, 2023. Following an unfavourable FDA decision on its ALS treatment, the stock hit an all-time low of $0.13 per share. Nethertheless, the stock experienced a significant rebound today, November 30th, 2023, rising by 26%, without accompanying press releases or SEC filings.
This resurgence prompts us to evaluate the possibilities for yet another company struggling to meet NASDAQ’s minimum bid requirements. A closer examination of recent months reveals intriguing developments that might pique the interest of potential investors. Our findings suggest BCLI holds promising near term prospects that could significantly alter its course.
Background:
BCLI specializes in developing therapies that utilize a patient’s own stem cells to combat severe neurodegenerative ailments like Amyotrophic Lateral Sclerosis (ALS) and progressive Multiple Sclerosis (MS). ALS affects nerve cells in the brain and spinal cord, while MS affects the central nervous system, causing problems with vision, balance, and muscle control.
The company holds exclusive global rights for the clinical advancement and commercialization of the NurOwn® technology platform, enabling the production of autologous MSC-NTF cells. These cells have received significant recognition and were awarded Orphan Drug designation by both the FDA and the European Medicines Agency (EMA).
Autologous MSC-NTF cells:
The way Autologous MSC-NTF cells work is very interesting. They’re a specific cell type obtained from an individual’s mesenchymal stem cells (MSCs). These cells are then genetically altered to produce neurotrophic factors (NTFs), which are designed to support and stimulate the growth and survival of nerve cells within the nervous system.
BrainStorm has conducted a Phase 3 clinical trial (NCT03280056) investigating the safety and effectiveness of multiple doses of autologous MSC-NTF cells for ALS. This trial received support from the California Institute for Regenerative Medicine, the ALS Association, and I AM ALS. Additionally, the company completed a Phase 2 open-label multicenter trial (NCT03799718) for progressive MS using these cells, backed by a grant from the National MS Society (NMSS).
Back to Square One:
Unfortunately for BCLI, 17 FDA advisory panel members voted that the data presented for their Phase 3 clinical trial for ALS did not demonstrate substantial evidence of the effectiveness of NurOwn for the treatment of mild-to-moderate ALS. There was simply only one member who voted in favour and another member that abstained from voting entirely.
Predictably, this resulted in a substantial downturn in the company’s share price, causing BCLI to plummet 87% below NASDAQ’s minimum bid compliance threshold of $1.00.
Subscribe to Microcapdaily.com Right Now by entering your Email in the box below.
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Is it Over?:
To those not well-versed in this matter, it might appear as if BCLI is forced to throw in the towel and start everything from scratch, but the reality is actually quite different.
When a drug fails in clinical trials, the pharmaceutical company or researchers typically reassess the data to understand why the drug failed.
They might conduct further analysis to determine if there were unforeseen side effects, issues with the study design, or if the drug simply didn’t show the desired effectiveness. They’ll work to make adjustments, refine the drug’s formulation, or in some cases, explore other alternatives by re-designing new clinical trials to evaluate the drug’s safety & efficacy for a different condition.
If none of those efforts yield a positive outcome, it’s typical to halt further development of that specific drug.
Regardless of the FDA’s decision, Co-CEO Lindborg mentioned, “We firmly believe that the data for NurOwn presented today provide a compelling case for approval, with clinical evidence in those with less advanced disease supported by strong and consistent biomarker data that are predictive of clinical response,”
Unfortunately for Lindborg, the decision does not rest with her. A panelist from the FDA, Lisa Lee, expressed, “Providing false hope can be ethically problematic and false hope is provided when the probability of a positive outcome is overestimated. And I think that seems to be the case here.”
After the devastating press release on September 27th, 2023, most of the retail crowd was on board with the FDA, mentioning it was time for BCLI to pack up, and go home – especially after seeing these comments from the FDA.
What Happened:
Amid FDA skepticism and investor uncertainties, BCLI took decisive action, securing a chance for a new phase 3 trial to showcase the effectiveness and safety of their NurOwn® technology for ALS treatment.
On October 24th, 2023, BCLI issued a press release affirming their conviction in the compelling data and expressing their intent to further substantiate it to the FDA.
More specifically, this was a “Strategic realignment” designed to:
- Support the company plans to conduct a double-blind, placebo-controlled Phase 3b U.S. clinical trial for NurOwn in ALS with an open-label extension and
- Continue to publish data from NurOwn’s Phase 3 clinical trial on biomarkers, long-term safety, survival, and the Expanded Access Program, providing transparency around NurOwn data and progressing ALS drug development.
Big Release:
Then on November 20th, 2023, BCLI issued another press release, stating that the FDA granted them an in person meeting to discuss the regulatory path forward for NurOwn® in ALS.
Within’ the last month or so, it appears the market is HIGHLY anticipating the outcome of this meeting, given it is now scheduled to take place on December 6, 2023.
This meeting allows BCLI to have a Special Protocol Assessment (SPA) with the FDA to agree on the overall protocol design for a confirmatory Phase 3 trial in ALS.
If you aren’t familiar with a SPA, it’s an agreement between a pharmaceutical company and the FDA regarding the design, endpoints, and size of a clinical trial intended to support a New Drug Application (NDA) or Biologics License Application (BLA). This agreement aims to confirm that the trial design, data analysis, and endpoints are acceptable to the FDA for the regulatory approval process.
A favorable result from this meeting could greatly benefit BCLI, paving the way for the actual launch of NurOwn®. This outcome stands in stark contrast to the prior belief, post the September 27th, 2023 news, which led many to assume otherwise.
If the trend remains positive, BCLI could see a significant surge in valuation, given the substantially reduced risk associated with a drug nearing commercial availability.
Conclusion:
Boosting the optimism for December 6th is the heightened enthusiasm among retail investors surrounding the company. Some speculate that the surge might mirror what SNGX experienced today, on November 20th, 2023. With technical day traders and swing traders in the mix, many perceive this as the final opportunity to join the trend before it gains further momentum. It’s crucial to remain vigilant as December 6 approaches rapidly, potentially ushering BCLI into an entirely altered and positive scenario.
We will update you on BCLI when more details emerge, subscribe to Microcapdaily to follow along!
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Disclosure: We have not been compensated for this article/video. MicroCap Daily is not an investment advisor; this article/video does not provide investment advice. Always do your research, make your own investment decisions, or consult with your nearest financial advisor. This article/video is not a solicitation or recommendation to buy, sell, or hold securities. This article/video is our opinion, is meant for informational and educational purposes only, and does not provide investment advice. Past performance is not indicative of future performance.
Picture by ckstockphoto from Pixabay.com
BioPharma
Apollomics (NASDAQ: APLM) Market Momentum: Pipeline Potential, Market Trends, and Retail Insights
Published
8 months agoon
November 27, 2023
Apollomics, Inc. (NASDAQ: APLM) has seen a significant surge in its shares, rising by ~20% at time of writing, accumulating a staggering 58% gain since November 21st, 2023. Surprisingly, there haven’t been any noticeable press releases or filings to explain this sudden valuation shift, so it’s prompted a closer examination on our end. Fortunately enough, we found the force driving the substantial gain, but first let’s look into APLM’s background to build a better picture of the opportunity at hand.
 Background:
Background:
APLM’s emergence as a public company is relatively recent, stemming from its merger with Maxpro Capital Acquisition Corp. (Nasdaq: JMAC) on March 30th, 2023. This merger also involved a $23.65 million private investment in public equity (PIPE) financing, a typical move when companies go public, aiming to secure capital. With this recent funding, APLM anticipates financial support through mid-2024, empowering the company to push forward with its ambitious lineup of 9 drug candidates.
APLM stands as an innovative player in the field of clinical-stage biopharmaceuticals, specializing in crafting cancer therapies that work hand-in-hand with the immune system and target precise molecular pathways to combat cancer. With a focus on oncology, the company boasts a lineup of nine potential drug candidates, six of which are actively progressing through clinical development stages.
Among its flagship projects are vebreltinib (APL-101), a potent c-MET inhibitor that targets multiple forms of non-small cell lung cancer (NSCLC), and uproleselan (APL-106), an E-Selectin antagonist designed to potentially enhance standard chemotherapy in treating acute myeloid leukemia.
Latest Release:
APLM recently announced significant news concerning its Chinese partner, Avistone Biotechnology, on November 11th, 2023. Avistone received conditional approval from China’s National Medical Products Administration (NMPA) to market vebreltinib. This medication is intended for treating a particular type of lung cancer known as MET exon 14 skipping non-small cell lung cancer (NSCLC).
Dr. Guo-Liang Yu, Apollomics’ leader, praised Avistone for this achievement, saying it’s a big step toward finding better treatments for tough cancers. Vebreltinib is a powerful medicine that targets how cancer cells grow. It could be a breakthrough for people with this specific lung cancer and other cancers caused by similar changes in cells.
Apollomics is talking with the U.S. Food and Drug Administration (FDA) about using vebreltinib to treat this kind of lung cancer in the U.S. They’re using data from their global study and Avistone’s study in China to do this.
It’s no secret, Lung cancer is a big problem, especially for people with non-small cell lung cancer (NSCLC), which is responsible for most lung cancer cases. This specific mutation has a significant unmet need, and APLM’s vebreltinib could be hope for those battling the devastating disease.
Subscribe to Microcapdaily.com Right Now by entering your Email in the box below.
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Market Opportunity:
To keep things simple, we’ll continue focusing on APLM’s Vebreltinib (APL-101), which is just 1 of 9 drugs in their pipeline.
This specific drug, cultivated through a collaboration with Beijing Pearl Biotechnology (now merged with Avistone Biotechnology), appears to display considerable potential. Its appeal lies in its demonstrated safety, effectiveness, and its potential to address a specific market segment where there’s a significant demand for treatments due to unmet medical needs.
Vebreltinib (APL-101):
APL-101 is being evaluated for its potential use in combating specific types of Non-Small Cell Lung Cancer (NSCLC), and a particularly aggressive form of brain cancer, secondary glioblastoma multiforme (sGBM).
APL-101 is a Type 1b class highly selective c-MET inhibitor. More simply put, it’s a drug that effectively targets and blocks the c-MET protein.
c-MET inhibitors have a wide potential range of markets due to the involvement of the c-MET pathway in various cancers and other diseases. The c-MET receptor, also known as the hepatocyte growth factor receptor (HGFR), plays a crucial role in cell growth, survival, and migration. Dysregulation of the c-MET pathway is associated with tumor growth, metastasis, and resistance to certain therapies in multiple cancer types.
Outside of NSCLC and sGBM, Apollomics has already seen promising effects inhibiting tumors related to certain types of cancers like gastric, hepatic, and pancreatic. But to keep things simple, we’ll stick to Apollomics core focus in NSCLC and sGBM.
The Market:
According to statistics published by the World Health Organization (WHO) from February 2022, 2.2 million patients were diagnosed with lung cancer in 2020, and non-small cell lung cancer accounted for nearly 85% of all lung cancer patients.
The global non-small cell lung carcinoma (NSCLC) market is expected to garner a market value of US$ 8.25 Billion in 2023 and is expected to accumulate a market value of US$ 21.40 Billion by registering a CAGR of 10% in the forecast period 2023 to 2033.
Delving deeper into particular types of NSCLC, the market potential for Exon-14 skip mutated NSCLC, c-MET amplifications in NSCLC (denovo), and c-MET amplifications in NSCLC (resistance driven) collectively represents a 3 billion-dollar opportunity.
In contrast, the market size for the population affected by epidermal growth factor receptor (EGFR) mutated NSCLC stands at a larger 7 billion-dollar opportunity.
Subscribe to Microcapdaily.com Right Now by entering your Email in the box below.
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
SPARTA Trial:
SPARTA is part of a larger study combining Phase 1 and Phase 2 trials, conducted in 13 countries, 90+ sites and 370+ patients worldwide. It aims to understand how safe and effective vebreltinib is in treating various cancers. More specifically, it’s looking at non-small cell lung cancer (NSCLC) with a specific genetic mutation called c-MET exon 14 skipping, as well as other cancers with c-MET amplification or fusions.
The trial involves different groups of patients: those new to treatments targeting the c-MET protein and those who have previously received such treatments for NSCLC with the mutation. Additionally, it includes patients with various solid tumors showing c-MET amplifications or fusions, including glioblastoma multiforme, a severe type of brain cancer.
The main goal of SPARTA is to measure how well vebreltinib works by assessing the overall response rate (ORR) and how long the response lasts (duration of response) using specific evaluation criteria for each type of tumor. Secondary goals involve looking at any side effects and additional measures like time to progression, progression-free survival, and overall survival.
Overall the clinical findings show that vebreltinib could be a promising new treatment for cancers caused by MET alterations, especially in Non-Small Cell Lung Cancer (NSCLC) with MetEx14 skipping mutation. In a phase 2 trial, there was 75% overall response rate in patients with advanced NSCLC.
If you’d like more formal clinical trial information for vebreltinib, it’s is available on clinicaltrials.gov under the Phase 1/2 SPARTA trial (NCT03175224).
Big Picture:
This is just 1 of 9 drugs in APLM’s pipeline, and APL-101 is just 1 of 2 key assets. With our initial examination of the company, we were taken aback by the comparatively low valuation despite the promising pipeline. Like many, there has been a considerable decline in share value since their public listing.
Now that China’s National Medical Products Administration (NMPA) has given conditional approval, the company intends to get Vebreltinib (APL-101) approved in the U.S. and has met with the FDA in July. With an existing approval by the NMPA, receiving the NDA Approval from the FDA should realistically be a much quicker process.
On top of this, more interesting developments have occurred from online sources, indicating substantial short activity, further hinting at the possibility of APLM being the next big short squeeze candidate.
What Happened & Retail Insights:
APLM seems to be gaining attention across various social media platforms, notibly Twitter and Reddit.
Numerous investors are discussing on different platforms that APLM might become a potential candidate for a short squeeze. Users have pointed out that the cost to borrow shares has risen significantly. This increased short activity seems to have also led to a shortage of available shares to borrow.
Increased buying activity is likely to intensify the pressure on short sellers, compelling them to buy back shares to cover their positions. Given today’s substantial surge in trading volume, short sellers may find themselves in a tight spot, potentially regretting their initial choice to wager against APLM.
We came across additional analysis (due diligence) about APLM on Reddit, providing concise insights. This is but one piece of content that’s drawing increased interest in the stock among retail investors.
APLM – Apollomics Inc- DD THREAD – BIO STOCK –
byu/StockProfilers inpennystocks
In recent times, Reddit has become a hub for discussions regarding stocks and potential market movements. It gained particular prominence in 2020 as a platform for detailed research and discussions about stocks, witnessed notably during the retail trading frenzy involving stocks like GME (GameStop) and AMC (AMC Entertainment Holdings).
Analyst Ratings:
Two analysts have set notably optimistic price targets for APLM, suggesting it might reach $21.00, representing a potential 1650% increase from its current value. Additional sources offer different projections, with estimates of $11.00 (an 816% increase), $14.00 (a 1066% gain), and $17.00 (a 1326% rise). It’s crucial to understand that analyst ratings are not certainties but rather projections based on their research. We commonly advise examining past coverage to assess the accuracy of analysts’ ratings and forecasts.
Conclusion:
In summary, APLM experienced a substantial surge, seemingly fueled by retail investors countering short sellers. However, beyond the retail-driven buzz speculating a potential short squeeze akin to TPST, a deeper narrative unfolds, signalling a compelling long-term proposition.
APLM’s pipeline exhibits fairly de-risked assets and promising data across multiple indications, not to mention large amount of partnership opportunities.
Analyst forecasts reflect a highly optimistic outlook, echoing positive retail sentiment. The ongoing SPARTA trial also unveils compelling data, suggesting a more profound story from a long term perspective.
Is APLM set for a short squeeze akin to TPST? Time will tell…. Nonetheless, within this realm, APLM seems fundamentally undervalued, making it a stock to keep a close eye on as more developments arise.
We will update you on APLM when more details emerge, subscribe to Microcapdaily to follow along!
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Disclosure: We have not been compensated for this article/video. MicroCap Daily is not an investment advisor; this article/video does not provide investment advice. Always do your research, make your own investment decisions, or consult with your nearest financial advisor. This article/video is not a solicitation or recommendation to buy, sell, or hold securities. This article/video is our opinion, is meant for informational and educational purposes only, and does not provide investment advice. Past performance is not indicative of future performance.
BioPharma
Processa Pharmaceuticals (NASDAQ: PCSA): Revolutionizing Cancer Treatment through Next-Generation Chemotherapies
Published
8 months agoon
November 23, 2023
Processa Pharmaceuticals’ (NASDAQ: PCSA) shares skyrocketed by over 260% from their October lows of $0.18 per share. Shares peaked at $0.65 on November 14th and more recently has consolidated around $0.43. The reason for this surge was not immediately clear given the lack of material events. However upon digging deeper, we’ve uncovered a very interesting aspect driving the sudden rise. Let’s take a closer look at their pipeline and company history to understand what potential developments might be on the horizon and whether the current levels are justifiable.
 Background:
Background:
PCSA is a clinical stage Pharmaceutical company that’s working to create better versions of chemotherapy that’s safer and more effective. More specifically called, “Next Generation Chemotherapy” or NGCs for short – they’re essentially just modified versions of existing FDA-approved oncology drugs.
The objective is of course to improve clinical outcomes and have less adverse events (AEs). They do this through altering the metabolism and distribution of the drug while maintaining the main mechanism that kills the actual cancer cells.
It’s all based off over 30 years of drug development expertise. Although efficacy and safety can vary drastically depending on the type of chemotherapy, it’s widely known that undergoing chemo is undeniably challenging for patients due to the potential negative impact on their well-being.
PCSA has a few clinical drugs that are based on Capecitabine, Gemcitabine, and Irinotecan.
Respective problems that occur with these drug treatments:
- Low treatment response with high side effect profile.
- High drug resistance and/or acquired resistance; administered as IV
- Significant side-effect profile limits dosing and drug use.
PCSA is actively working to achieve:
- Actively minimizing Adverse Events (AEs), consequently broadening the patient base.
- Developing an oral therapy that accelerates the action of cancer-killing molecules while enhancing their quantity to mitigate drug resistance.
- Ensuring that cancer-killing molecules selectively enter cancer cells rather than normal cells, enhancing effectiveness and reducing toxicity.
Subscribe to Microcapdaily.com Right Now by entering your Email in the box below.
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
What Happened:
We took a closer look at PCSA’s SEC filings and found something interesting: in October, there were a lot of Form 4 submissions. This seems to be the main reason for the sudden increase in PCSA’s valuation. Between October 10th and October 12th, insiders bought over 590,000 shares, and it wasn’t just one person—several insiders made these purchases. More often than not, this suggests a high expectation for something big happening soon.
Management Team:
Another important aspect to consider is the leadership team. It’s a fundamental step in conducting your due diligence since past achievements and expertise often pave the way for future success. Let’s swiftly overview just two key individuals in the company. For more information on others, click here.
George Ng | CEO:
Mr. Ng is a highly experienced figure in the life sciences industry with an extensive career involving founding and leading various companies. Notably, at Scilex Pharmaceuticals, Inc. (now, Scilex Holding Co (NASDAQ:SCLX)), where he played a key role in the company’s journey from development and clinical trials to NDA submission, FDA approval, a substantial $140 million financing round, the successful commercial launch of their first FDA-approved drug product, and the ultimate sale of the company. His career history includes a range of senior management positions across globally recognized biotechnology and pharmaceutical companies like Sorrento Therapeutics, Inc. (NASDAQ: SRNE), BioDelivery Sciences International, Inc. (NASDAQ: BDSI), Spectrum Pharmaceuticals, Inc. (NASDAQ: SPPI), and Alpharma, Inc. (NYSE:ALO), which is now part of Pfizer Inc. In these roles, Mr. Ng played pivotal roles in strategy, development, fundraising, and the commercialization of multiple pharmaceutical drug products.
David Young | President, Research & Development:
Dr. Young possesses extensive expertise in pharmaceutical research and development, spanning over three decades. As the Chief Scientific Officer of Questcor Pharmaceuticals from 2009 to 2014, he collaborated with the FDA to modernize Acthar Gel’s label and secure FDA approval for Infantile Spasms. His impactful involvement contributed significantly to Questcor’s transformation from near bankruptcy in 2007 to an esteemed orphan drug specialty company, reaching a valuation of around $5.6 billion by 2014. He was also the Founder and CEO of GloboMax LLC, a CRO specializing in FDA drug development that was purchased by ICON plc in 2003. Preceding his tenure at GloboMax, Dr. Young held a tenured Associate Professor position at the University of Maryland School of Pharmacy, leading a team of 30 individuals dedicated to evaluating the biological properties of drugs and drug delivery systems in both animals and humans.
Young’s experience extends even further: he served on FDA Advisory Committees, played a key role in an FDA-funded Clinical Pharmacology contract, oversaw the evaluation of oral products in a UMAB-FDA contract leading to FDA Guidance improvements, educated FDA reviewers for five years, participated in NIH grant review committees, and co-led a National Cancer Institute contract evaluating new oncology drugs.
Overall, Young’s met more than 100 times with the FDA on more than 50 drug products and played a vital role in over 30 NDA/supplemental NDA approvals. Additionally, he has over 150 published works, including presentations to the FDA, and numerous talks at scientific and investment gatherings.
It’s certainly significant that someone with such vast experience as Dr. Young is associated with PCSA, valued at ~10M at time of writing. Considering this executive’s extensive background in navigating FDA approvals, we hold a strong positive outlook on its potential trajectory. Dr. Young’s substantial insider purchases also strongly hint at positive developments ahead.
Subscribe to Microcapdaily.com Right Now by entering your Email in the box below.
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Phase 1B Trial Update:
The last Phase 1B trial update is written in a fairly scientific format, so we’ve taken the liberty of simplifying the release to better your understanding.
The Phase 1B trial of NGC-Cap in patients with gastrointestinal cancer aims to create a personalized treatment method that could be safer and more effective.
NGC-Cap is a treatment that mixes PCS6422, which stops an enzyme called DPD, with small amounts of a common chemotherapy drug, capecitabine. This chemotherapy drug turns into 5-FU in the body. DPD helps change 5-FU into another substance called FBAL, which usually causes strong side effects, making it tough to give higher doses of the chemotherapy treatment.
Processa discovered that regularly checking DPD levels, (shown by the substance FBAL), could help understand how each patient reacts to different NGC-Cap doses. This information might help doctors create specific treatment plans for each patient, aiming to reduce side effects and make the treatment work better while keeping the patient safe and comfortable.
Capecitabine is among the most widely used chemotherapy drugs and is dosed based on a standard dosage regimen for all patients. That said, many patients cannot tolerate that dose and must either have their dose reduced or have their treatment interrupted, which means ultimately means it’s less effective overall.
The discoveries made in this interim data is very promising and could mean that PCSA is now capable of personalized treatment plans, helping patients potentially handle the treatment much better without AEs.
Key Catalysts and Considerations:
There’s supposedly more updates soon for PCSA’s two main drugs, PCS6422/NGC-Cap and PCS3117/NGC-Gem.
For PCS6422/NGC-Cap, they’re finishing up the Phase 1B study and planning the Phase 2 trial based on feedback from the FDA.
With PCS3117/NGC-Gem, they’re working closely with the FDA to plan the drug’s development and how it will be tested.
There’s also been a recent change in leadership, bringing in a new CEO, George Ng in August 2023. This new leader has experience in turning companies around, developing business, and as mentioned, knows a lot about cancer treatment. The company is also considering licensing non-core assets to bring in money without dilution.
Overall PCSA’s new chemotherapy drugs look promising for treating a diverse range of cancers, meaning a potential billion-dollar US market opportunity alone. They have experienced leaders who know how to get medicines approved and make successful deals.
As of June 2023, they have about $8.7 million in cash, meaning a runway into the second half of 2024. They’re also thinking looking into potential partnership opportunities to further to develop their pipeline to avoid dilution.
Some of these events would be considered significant catalysts, potentially propelling the company to new heights. With a relatively low float, price fluctuations could be volatile in nature—leading to substantial gains or complete loss of investment. However at these levels, PCSA certainly seems like an interesting opportunity for potential upside, be sure to keep them on your radar.
We will update you on PCSA when more details emerge, subscribe to Microcapdaily to follow along!
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Disclosure: We have not been compensated for this article/video. MicroCap Daily is not an investment advisor; this article/video does not provide investment advice. Always do your research, make your own investment decisions, or consult with your nearest financial advisor. This article/video is not a solicitation or recommendation to buy, sell, or hold securities. This article/video is our opinion, is meant for informational and educational purposes only, and does not provide investment advice. Past performance is not indicative of future performance.
Recent Posts

Clean Vision Corp (OTC: CLNV): Overcoming the Plastic Waste Crisis

Meta Materials (NASDAQ: MMAT): More Due Diligence and Exploring Latest Developments

Integrated Cannabis Solutions’ (OTC: IGPK) 633% Surge: Exploring Catalysts, Company Overview, and Growth Potential in 2024

Sonoma Pharmaceuticals (NASDAQ: SNOA): Potential Surge to Speculations – What Lies Ahead?

1847 Holdings (NYSE: EFSH) Soars: Insights, Acquisitions, and What Lies Ahead
Trending
-

 Uncategorized2 years ago
Uncategorized2 years agoMeta Materials Inc (OTCMKTS: MMTLP) Enormous Short Position in Trouble as Next Bridge Hydrocarbons Set to Stop Trading (George Palikaras & John Brda on Corporate Action)
-

 Micro Cap Insider3 years ago
Micro Cap Insider3 years agoMedium (KOK PLAY) The Parabolic Rise of Metal Arts (OTCMKTS: MTRT)
-

 Media & Technology3 years ago
Media & Technology3 years agoHealthier Choices Management Corp. (OTCMKTS: HCMC) Powerful Comeback Brewing as PMI Patent Infringement Lawsuit Moves Forward
-
Media & Technology4 years ago
AMECA Mining RM; the Rise of Southcorp Capital, Inc. (OTCMKTS: STHC)
-

 Media & Technology3 years ago
Media & Technology3 years agoSNPW (Sun Pacific Holding Corp) Power Brewing: 50MW solar farm project in Durango Mexico MOU with Atlas Medrecycler 48,000 SF New Partnership Queensland Australia Solar Farm.
-

 BioPharma2 years ago
BioPharma2 years agoAsia Broadband (OTCMKTS: AABB) On the Move Northbound Since Sub $0.08 Dip as Crypto Innovator Elevates AABB Crypto Exchange & Enters the NFT Space
-

 Uncategorized2 years ago
Uncategorized2 years agoMeta Materials Inc (OTCMKTS: MMTLP) Short Squeeze S-1a4 Filing Signals S1 Approval Could Be Days Away (Next Bridge Hydrocarbons Spin-Off)
-
 BioPharma3 years ago
BioPharma3 years agoHumbl Inc (OTCMKTS: HMBL) Major Reversal as Powerful Advisor Rejoins the Team & Looks to Uplist to Major Exchange