Uncategorized
Major Run on RLFTF (Relief Therapeutics Holding) On Successful ZYESAMI 2b/3 Trial Results as Submission of Phase 3 data & EUA Looms Large
Published
3 years agoon
By
Boe Rimes
RLFTF has been moving northbound in a hurry after its partner NeuroRx, Inc. announced the Phase 2b/3 trial* of ZYESAMI™ (aviptadil, previously RLF-100™) for the treatment of Respiratory Failure in critically ill patients with Covid-19 has demonstrated multidimensional benefit around its prespecified primary endpoint of Recovery from Respiratory Failure with discharge from hospital and ICU (without relapse) by day 28 in patients with critical Covid-19 who were treated with High Flow Nasal Oxygen. NeuroRx has signed an agreement to complete a business combination with Big Rock Partners Acquisition Corporation (NASDAQ:BRPA). ZYESAMI™ represents a huge step forward in the fight against covid 19 and EU seems likely now and could be the catalyst that finally ignites RLFTF and propels the stock into a whole new level. Investors are looking for a break over $0.95 highs seen in August for confirmation of the next leg up.
ZYESAMI™ a synthetic vasoactive intestinal peptide (VIP) is the first COVID-19 therapeutic to demonstrate the ability to block replication of the SARS-CoV-2 virus in human lung cells and monocytes, while also preventing synthesis of cytokines in the lung. While drugs such as dexamethasone, Remdesivir and Monoclonal antibodies/convalescent plasma have been approved for fighting COVID, these drugs have minimal efficacy, are expensive to produce and difficult to transport. ZYESAMI™ is simple to manufacture and cheap to produce at scale. Another popular synthetic vasoactive intestinal peptide (VIP) is insulin. ZYESAMI™ represents a significant breakthrough in the fight against covid 19 and means there is now an effective therapy for patients in the ICU improving oxygenation, and getting patients out of the hospital faster. Rapid adoption in ICUs nationwide can be anticipated as well as peer reviewed publication of results in the Lancet and other journals. NeuroRx is planning to file for Emergency Use Authorization in this patient population if positive results continue to be demonstrated at day-60 endpoint in line with FDA’s new guidance. Based on the favorable results, one can reasonably conclude the submission of phase 3 data has already occurred to the FDA in anticipation of possible EUA.
 Relief Therapeutics Holding AG (SIX: RLF; OTCQB: RLFTF) focuses primarily on clinical-stage programs based on molecules of natural origin (peptides and proteins) with a history of clinical testing and use in human patients or a strong scientific rationale. Currently, Relief is concentrating its efforts on developing new treatments for respiratory disease indications. Its lead drug candidate RLF-100™ (aviptadil) is being investigated in two placebo-controlled U.S. late-stage clinical trials in respiratory deficiency due to COVID-19. Relief holds a patent issued in the United States and various other countries covering potential formulations of RLF-100™. The Company’s partner NeuroRx draws upon more than 100 years of collective drug development experience from senior executives of AstraZeneca, Eli Lilly, Novartis, Pfizer, and PPD. In addition to its work on ZYESAMI™, NeuroRx has been awarded Breakthrough Therapy Designation and a Special Protocol Agreement to develop NRX-101 in suicidal bipolar depression and is currently in Phase 3 trials.
Relief Therapeutics Holding AG (SIX: RLF; OTCQB: RLFTF) focuses primarily on clinical-stage programs based on molecules of natural origin (peptides and proteins) with a history of clinical testing and use in human patients or a strong scientific rationale. Currently, Relief is concentrating its efforts on developing new treatments for respiratory disease indications. Its lead drug candidate RLF-100™ (aviptadil) is being investigated in two placebo-controlled U.S. late-stage clinical trials in respiratory deficiency due to COVID-19. Relief holds a patent issued in the United States and various other countries covering potential formulations of RLF-100™. The Company’s partner NeuroRx draws upon more than 100 years of collective drug development experience from senior executives of AstraZeneca, Eli Lilly, Novartis, Pfizer, and PPD. In addition to its work on ZYESAMI™, NeuroRx has been awarded Breakthrough Therapy Designation and a Special Protocol Agreement to develop NRX-101 in suicidal bipolar depression and is currently in Phase 3 trials.
ZYESAMI™ a synthetic vasoactive intestinal peptide (VIP), is the first COVID-19 therapeutic to demonstrate the ability to block replication of the SARS-CoV-2 virus in human lung cells and monocytes, while also preventing synthesis of cytokines in the lung. Since July 2020, severe COVID-19 patients have been treated with RLF-100TM under U.S. FDA Emergency Use Investigational New Drug (IND) authorization and Expanded Access Protocol authorization for the treatment of respiratory failure in COVID-19. Relief also holds a patent issued in the United States and various other countries covering potential formulations of RLF-100TM. VIP is shown to block Coronavirus replication in the ATII cell, block cytokine synthesis, block viral-induced cell death (cytopathy), and upregulate surfactant production. To our knowledge, other than ZYESAMI™, no currently proposed treatments for Covid-19 specifically target these vulnerable Type II cells. Recent laboratory findings suggest that VIP directly interferes with the spike protein complex of the SARS-CoV-2 virus.
On February 23 NeuroRx, Inc. announced the Phase 2b/3 trial* of ZYESAMI™ (aviptadil, previously RLF-100™) for the treatment of Respiratory Failure in critically ill patients with Covid-19 has demonstrated multidimensional benefit around its prespecified primary endpoint of Recovery from Respiratory Failure with discharge from hospital and ICU (without relapse) by day 28 in patients with critical Covid-19 who were treated with High Flow Nasal Oxygen. Although not envisioned at the start of the clinical trial, High Flow Nasal Oxygen has become the predominant form of treatment in Covid-19 respiratory failure, with mechanical ventilation reserved for those whose blood oxygen levels cannot be maintained on this less invasive modality. The trial was conducted at 10 U.S. hospitals under the direction of NeuroRx in collaboration with RELIEF THERAPEUTICS Holding AG (SIX: RLF; OTCQB: RLFTF). NeuroRx has signed an agreement to complete a business combination with Big Rock Partners Acquisition Corporation (NASDAQ:BRPA).
Investor sentiment in RLFTF is very high:
$RLFTF CLIMBING NOW behind HEAVY VOLUME!!!!! Could be last opp to get in cheap
— Crazy Cowboy Trader (@crookedneck) February 24, 2021
$RLFTF Covid play https://t.co/fx0JedJNRL
— RSG (@rssenn_s) February 24, 2021
To Find out the inside Scoop on Relief Subscribe to Microcapdaily.com Right Now by entering your Email in the box below
The clinical trial was originally approved as a 28-day study at FDA’s direction. In December, NeuroRx added a 60-day endpoint based on the recognition that the traditional 28-day endpoint adopted in the 1990s for trials in Acute Respiratory Distress Syndrome is not appropriate for critically ill patients with Covid-19, who are frequently maintained in the ICU with advanced technologies well beyond this time point. NeuroRx and other clinical trial sponsors alerted FDA to this trend and yesterday the FDA published formal guidance† changing the required time for measuring the prespecified endpoint of “alive and free of respiratory failure” in critically ill patients to 60 days. Interim data are being reported because they were unblinded as per the original protocol and the last patient in the trial reached day 60 yesterday. Therefore, study conduct cannot be adversely influenced by release of these interim findings.
 At 28 days, patients treated with ZYESAMI™ demonstrate 35% higher likelihood of recovery from respiratory failure with continued survival compared to patients treated with placebo (Hazard Ratio 1.53; P=.08). In tertiary care hospitals, ZYESAMI-treated patients were 46% more likely to recover and return home before day 28 (Hazard Ratio controlling for age and severity 1.84; P=.058). Should these trends continue through day 60, they have the potential to reach statistical significance. At day 28, a highly significant 10-day difference in median time to recovery and hospital discharge has emerged in ZYESAMI-treated patients compared to those treated with placebo (P<.006).
At 28 days, patients treated with ZYESAMI™ demonstrate 35% higher likelihood of recovery from respiratory failure with continued survival compared to patients treated with placebo (Hazard Ratio 1.53; P=.08). In tertiary care hospitals, ZYESAMI-treated patients were 46% more likely to recover and return home before day 28 (Hazard Ratio controlling for age and severity 1.84; P=.058). Should these trends continue through day 60, they have the potential to reach statistical significance. At day 28, a highly significant 10-day difference in median time to recovery and hospital discharge has emerged in ZYESAMI-treated patients compared to those treated with placebo (P<.006).
Should the above trends continue through day 60, NeuroRx anticipates filing a request for Emergency Use Authorization in this population of critically ill patients (i.e. those on High Flow Nasal Oxygen) who have exhausted all currently approved treatments. FDA decisions implement a benefit/risk framework. NeuroRx previously announced the high degree of safety observed with use of ZYESAMI. This safety has continued to be documented in the more than 300 additional patients treated under the Expanded Access Protocol and in patients who have filed requests under the federal Right to Try act.
The study’s principal investigators, Dushyantha Jayaweera, M.D., FACP (University of Miami), Professors J. Georges Youssef, M.D. (Houston Methodist Hospital), and Richard Lee, M.D. (University of California, Irvine), commented, “We are excited to report that ZYESAMI demonstrates a highly significant reduction in time to recovery compared to patients treated with placebo in those treated with High Flow Nasal Oxygen, together with increased likelihood of recovery and excellent safety. We look forward to learning whether this benefit can also be shown for patients treated with other stages of Covid-19 with inhaled forms of ZYESAMI. We look forward to working with the sponsor to secure emergency use authorization for ZYESAMI in this population of patients.”
https://twitter.com/TonyFauci2/status/1364294025675755522
For more on Relief Therepuetics Subscribe Right Now!
RLFTF has been moving northbound in a hurry after its partner NeuroRx, Inc. announced the Phase 2b/3 trial* of ZYESAMI™ (aviptadil, previously RLF-100™) for the treatment of Respiratory Failure in critically ill patients with Covid-19 has demonstrated multidimensional benefit around its prespecified primary endpoint of Recovery from Respiratory Failure with discharge from hospital and ICU (without relapse) by day 28 in patients with critical Covid-19 who were treated with High Flow Nasal Oxygen. NeuroRx has signed an agreement to complete a business combination with Big Rock Partners Acquisition Corporation (NASDAQ:BRPA). ZYESAMI™ represents a huge step forward in the fight against covid 19 and EU seems likely now and could be the catalyst that finally ignites RLFTF and propels the stock into a whole new level. Investors are looking for a break over $0.95 highs seen in August for confirmation of the next leg up. ZYESAMI™ a synthetic vasoactive intestinal peptide (VIP) is the first COVID-19 therapeutic to demonstrate the ability to block replication of the SARS-CoV-2 virus in human lung cells and monocytes, while also preventing synthesis of cytokines in the lung. While drugs such as dexamethasone, Remdesivir and Monoclonal antibodies/convalescent plasma have been approved for fighting COVID, these drugs have minimal efficacy, are expensive to produce and difficult to transport. ZYESAMI™ is simple to manufacture and cheap to produce at scale. Another popular synthetic vasoactive intestinal peptide (VIP) is insulin. ZYESAMI™ represents a significant breakthrough in the fight against covid 19 and means there is now an effective therapy for patients in the ICU improving oxygenation, and getting patients out of the hospital faster. Rapid adoption in ICUs nationwide can be anticipated as well as peer reviewed publication of results in the Lancet and other journals. NeuroRx is planning to file for Emergency Use Authorization in this patient population if positive results continue to be demonstrated at day-60 endpoint in line with FDA’s new guidance. Based on the favorable results, one can reasonably conclude the submission of phase 3 data has already occurred to the FDA in anticipation of possible EUA. We will be updating on Relief on a daily basis so make sure you are subscribed to microcapdaily.com so you know what is going on with Relief.
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Disclosure: we hold no position in Relief either long or short and we have not been compensated for this article.
You may like
-


Relief Therapeutics Holding AG (OTC: RLFTF) Big Move as NeuroRx. (NRXP) Reports ZYESAMI™ Met Primary Endpoint in its Covid-19 Phase 2b/3 Trial
-


Powerful Run on Relief Therapeutics Holding SA (SWX: RLF) (OTCQB: RLFTF) Phase 2b/3 Trial Top Line Data Coming & ZYESAMI™ (RLF-100TM: aviptadil) Trials
-
Relief Therapeutics Hldg AG (OTC: RLFTF) Gaining as RLF-100 (aviptadil) Top Line Data Looms
-


Relief Therapeutics Holding AG (SWX: RLF) (OTCQB: RLFTF) RLF-100™ (Aviptadil); a Synthetic VIP That Works
-


Comeback Time for Relief Therapeutics Holding AG (OTCMKTS: RLFTF): RLF-100 (aviptadil)
-
Relief Therapeutics Holding SA (OTCMKTS: RLFTF) RLF-100 (aviptadil) The Bull Has Awoken
Featured
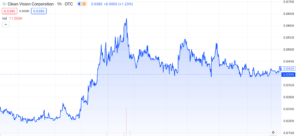
Clean Vision Corp (OTC: CLNV): Overcoming the Plastic Waste Crisis
Published
6 months agoon
January 18, 2024
Clean Vision Corporation (OTC: CLNV) has experienced several interesting developments recently, but it hasn’t noticeably influenced the market with any substantial gains. Nonetheless, we believe it’s worth providing an update on the company given it’s been a few months since our last mention. In today’s discussion, we’ll explore a variety of updates and their significance, with aim of providing insight on what to expect for 2024.
Background:
Clean Vision is led by Dan Bates, and their goal is to tackle the global plastic waste crisis head-on. Their wholly owned subsidiary, Clean Seas, has developed the Plastic Conversion Network (PCN), a groundbreaking technology aimed at diverting millions of tons of waste plastic from landfills, incineration, and oceans. The PCN converts this plastic feedstock into clean fuels and green hydrogen, significantly reducing reliance on fossil fuels and lowering the carbon footprint.
For a brief 2 minute overview on the company, feel free to reference the video CLNV’s subsidiary put together on YouTube. Here’s the link.
Clean Seas utilizes proven pyrolysis technology to produce environmentally friendly products, which are sold to multinational petrochemical companies, driving the circular plastic economy. Operational PCN facilities are already in place in Morocco and India, with additional conversion facilities in development across West Virginia, Arizona, and Southeast Asia. Long-term feedstock supply agreements exceeding one million tons of waste plastic annually have been secured at no cost.
Their recently trademarked brand, AquaH®, is produced in their PCN. According to the release, it offers a differentiated green hydrogen product from carbon-neutral sources. Currently, hydrogen is predominantly produced through methods that involve fossil fuels, which of course contributes to global carbon emissions. Furthermore according to Deloitte’s 2023 global green hydrogen outlook, this could be a $1.4T annual market by 2050.
$65 Million Plastic Conversion Facility:
CLNV is making big moves in West Virginia and according to the release on October 24th, 2023, they’ve brought in some serious players—CDI Engineering Solutions and ERM—to help out with their Clean-Seas West Virginia project.
CDI has over 70 years of experience integrating engineering, design, project support, procurement and construction management services to the energy, chemicals and electrical infrastructure markets.
ERM is the world’s largest advisory firm focused solely on sustainability, offering environmental, health, safety, risk and social expertise for more than 50 years with more than 8,500 dedicated professionals operating across 40 countries.
The plan is to kick things off in 2024, turning 100 tons of plastic every day into recycled plastics and clean fuels. It’s a hefty project with a $65 million investment, creating over 200 jobs initially. And they’re not stopping there—they want to scale up to 500 tons of plastic per day over time.
West Virginia Governor Jim Justice is also on board, throwing over $12 million in state incentives to support the project.
Governor Jim Justice made a reference to Clean Seas in his state of the union address. If you want to catch the mention, go to 34:15 in the video. The three minutes leading up to it are also worth reviewing.
Subscribe to Microcapdaily.com Right Now by entering your Email in the box below.
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Launches Global Operations:
CLNV made another significant advancement, planning to launch waste plastic conversion facilities in the European Union, Eastern Europe, and Southeast Asia. This will be accomplished through their new subsidiary, Clean-Seas Partners UK Limited (CS-UK), who of course shares the same vision of creating sustainable solutions to the global plastic pollution crisis.
Under the leadership of Managing Director Shaun Wootton, CS-UK will play a crucial role in strategic project development and investment facilitation, leveraging established relationships in the Middle East, Southeast Asia, and Europe.
To fortify effective governance and strategic direction, CS-UK is assembling a distinguished board with internationally recognized figures in banking, sustainability, and energy. This approach aims to have a diverse and experienced board guiding CS-UK in realizing its vision of promoting sustainability and environmental stewardship across diverse regions.
$340 Million Bond Offering:
CLNV even announced they partnered with a global advisory firm, Grant Thornton, to issue up to $340 million in Green Bonds. This is the world’s sixth-largest network of independent accounting and consulting firms, employing 62,000 people in more than 130 countries and had revenues of $6.6 billion in 2021. These bonds will fund the expansion of Clean Vision’s Plastic Conversion Network (PCN) under the “Clean-Seas” initiative worldwide, aimed at combatting plastic pollution on a global scale.
With the Green Bond’s net proceeds, CLNV plans to deploy at least six plastic waste conversion lines globally, with strategic locations in West Virginia, Arizona, Southeast Asia, and expansion in Morocco. The Green Bond is also expected to attract environmentally conscious investors, setting a new standard for corporate responsibility.
$15M Government Loan:
Lastly, under the capable management of Huntington Bank, CLNV has recently secured a $15 million government loan. What sets this apart is that the loan is FORGIVABLE.
A forgivable loan is a type of loan where the borrower is not required to repay the borrowed amount under certain conditions. Typically, these conditions are related to the borrower meeting specific criteria, such as using the funds for approved purposes, maintaining certain employment levels, or achieving predetermined goals. If the borrower fulfills these conditions, the loan is forgiven, and they are not obligated to repay the borrowed amount. Forgivable loans are often used as an incentive or support for specific activities, such as job creation, small business development, or other initiatives that contribute to economic growth or community welfare.
Not to mention it won’t result in any dilution for shareholders. This is an unexpected and uncommon accomplishment for an OTC company. Securing a government loan of this size without any dilution is truly impressive.
Conclusion:
CLNV has made impressive strides tackling the global plastic waste crisis, especially given their valuation of merely $22.65 million. The team has swiftly achieved key objectives, including a $65 million plastic conversion facility in West Virginia, global expansion through Clean-Seas Partners UK Limited, a $340 million Green Bond Offering, and a remarkable $15 million forgivable government loan. The vast $1.4 trillion market they’re tapping into offers an interesting opportunity with current indicators looking positive. Nevertheless, it’s crucial to acknowledge that there is still significant work ahead, and the team needs to maintain consistent execution to turn this potential into a reality.
We will update you on CLNV when more details emerge, subscribe to Microcapdaily to follow along!
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Disclosure: We have not been compensated for this article/video. MicroCap Daily is not an investment advisor; this article/video does not provide investment advice. Always do your research, make your own investment decisions, or consult with your nearest financial advisor. This article/video is not a solicitation or recommendation to buy, sell, or hold securities. This article/video is our opinion, is meant for informational and educational purposes only, and does not provide investment advice. Past performance is not indicative of future performance.
Picture by pasja1000 from Pixabay
Featured
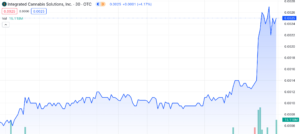
Integrated Cannabis Solutions’ (OTC: IGPK) 633% Surge: Exploring Catalysts, Company Overview, and Growth Potential in 2024
Published
7 months agoon
January 12, 2024
Integrated Cannabis Solutions (OTC: IGPK) has undergone a remarkable uptrend, surging an impressive 633% since December 11th, 2023, with 166% of that surge taking place across yesterday’s trading session and today, January 11th, 2023. Both days have been marked by unprecedented volume – Yahoo Finance reported an almost 30x increase, with 115,867,027 shares traded by close yesterday. We’re already seeing 90,092,317 shares traded this morning and it’s just barely noon. Today we’ll explore the catalysts behind the surge, offer a comprehensive overview of the company, and evaluate IGPK’s potential for sustained growth throughout 2024.
Background:
Let’s get straight to it. IGPK is the result of a recent reverse merger with Integrated Cannabis Solutions and JFH Digital E-Commerce Corp. The first thing you’ll notice is finding the website isn’t a walk in the park, we’re fairly certain there isn’t one yet, at least one that will help in any way related to more investment information. Your best bet for more any information is to check out IGPK’s OTC Market page for details, but even the company description on there is not accurate. We’ve mainly found the following information through filings, IGPK’s Twitter, and other online users.
Keep in mind this breakdown might not be flawless given we’re piecing it together mostly from what folks on X are saying. But we’ll try our absolute best to lay it all out for you.
IGPK appears to have been a shell for little while until JFH stepped in. A user on X, @stockplayer30, broke it down fairly simply, stating that the shell’s slate was wiped clean, cancelling all notes payable and any debt. Whether it’s a promissory note, convertible note, or convertible debenture, the main point is they ditched all debt. JFH has an opportunity to start fresh, and it certainly makes this deal a lot more interesting.
Just a heads up, it’s a Chinese merger. If the idea of a Chinese leadership team makes you a bit wary, you might want to pause here. However if you were in the trading game during the summer of ’23, you probably remember those crazy spikes in some Chinese Nasdaq deals. And get this – no big press releases or SEC filings to explain those sudden jumps either.
Subscribe to Microcapdaily.com Right Now by entering your Email in the box below.
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Company Description:
Onto what the company actually does. According to @SuperRobotOTC on X, this is a digital e-commerce company based in China, here’s a link to their e-commerce website. This user also put together a great overview of the company on YouTube, if you’d like to watch something informative in video format, click here.
Another user, @igal_n, found a blurb on the company that states, “Junfenghuang (JFH) is a digital asset. It is a token issued by Uplus Future Company with the help of blockchain technology. It has no direct relationship with the original equity”.
The Potential:
What makes this story extremely interesting is the sheer magnitude of how large JFH is, the intrinsic value does not appear to be valued accurately in the market, given it’s only freshly merged into IGPK’s tiny shell company on the OTC.
Their Gross Merchandise Value (GMV) is heading north of 50 billion yuan, and post-merger profits from service outlets are looking at a hefty 10 billion yuan – yes, billion with a B.
Steering the ship is a leadership team featuring President Wang Dejun, Treasurer Xie Weiji, and Director Yang Lanfang. With a whopping 750 subsidiaries, 250,000 merchants, and 30 million registered users. We’ve also heard from other sources that the registered users could be nearly double that, coming in at 50 million registered users.
These numbers are substantial for a company with a $7 million market cap. Looking ahead, it won’t be shocking if IGPK sets its sights on moving up to a bigger exchange like NASDAQ. It’s no secret they’re already in the big leagues – or it at least appears so. If that were the case, they’d of course have enhanced credibility, more visibility, and increased access to capital with institutional funding.
The App:
Now, you might be wondering, “Sounds cool, but it’s a Chinese merger with a whole setup on the other side of the planet. Can we trust this info?” @SuperRobotOTC has also gone the extra mile by downloading the app, and gave us the lowdown in video format. On top of the SEC filings, this is an added layer of trust & credibility we can attribute to this new venture. Here’s the link to the video.
Conclusion:
Fortunately it appears IGPK is still for the most part flying under the radar. There’s not even a proper website or accurate update on IGPK’s OTC Market overview to tell us what the company even entails. But here’s the silver lining – that might mean you’re still early. The intrinsic value of IGPK appears strongly disproportionate to its current value in the market.
Our advice? Keep a close eye on IGPK’s journey as it takes on this exciting phase of growth and exploration. It’s likely this story will catch wind quickly and it could be a great time to take advantage. As @SuperRobotOTC eluded to in his video, this could be the OTC’s largest merger, with a potential $70B valuation.
We will update you on IGPK when more details emerge, subscribe to Microcapdaily to follow along!
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Disclosure: We have not been compensated for this article/video. MicroCap Daily is not an investment advisor; this article/video does not provide investment advice. Always do your research, make your own investment decisions, or consult with your nearest financial advisor. This article/video is not a solicitation or recommendation to buy, sell, or hold securities. This article/video is our opinion, is meant for informational and educational purposes only, and does not provide investment advice. Past performance is not indicative of future performance.
Picture by geralt from Pixabay
Featured
Orchard Therapeutics’ (NASDAQ: ORTX) Shares Skyrocket: How To Predict Biotech M&A
Published
10 months agoon
October 5, 2023
Orchard Therapeutics (NASDAQ: ORTX) experienced a remarkable 97% surge in its shares following a major announcement on October 5th, 2023. The renowned global gene therapy leader, Orchard, has been successfully acquired by Kyowa Kirin (OTC: KYKOF), solidifying its position as a fully owned subsidiary.
The Agreement:
The agreement outlines that Kyowa plans to buy all Orchard Therapeutics’ shares at $16.00 per share in cash (totalling about $387.4 million or around ¥57.3 billion) upon closure. This price is a 144% premium to Orchard’s previous value at close on October 4th, 2023.
As part of this deal, Orchard shareholders will receive an additional non-transferable CVR. Holders of the CVR will get a cash payout of $1.00 per share once OTL-200 for treating MLD in the U.S. gains approval.
For those that aren’t familiar, CVR stands for Contingent Value Right. It is a type of financial instrument or contractual right that entitles the holder to a payment or benefit if specific, predefined events or conditions are met. In the context of mergers or acquisitions, CVRs are often used as an additional incentive or compensation to shareholders based on the performance or success of certain agreed-upon benchmarks, such as the approval of a drug, achieving specific sales milestones, or reaching certain financial targets. The CVR allows shareholders to participate in the potential future success of a merged or acquired entity.
Subscribe to Microcapdaily.com Right Now by entering your Email in the box below.
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Predicting M&A in Advance:
Now that Orchard has been acquired, it’s probably not in your best interest to be trading it. What’s more important is being able to spot potential merger & acquisitions (M&A) before it happens. While it may seem like a large hurdle, enough research can put you in the right spot at the right time. And if you can do it well, there’s no question you’ll be increasing your earnings. Here’s 3 factors to think about when looking for potential M&A candidates.
1. Misvalued to the Extreme: Celgene’s Journey
Ever noticed how stock prices often have a mind of their own? Let’s talk about Celgene ($CELG), a big shot in the biotech world. Riding high with a super successful blood cancer drug called Revlimid, its stock price shot up from $25 to an incredible $139 between 2010 and 2017. Investors were all in.
But out of nowhere everything shifted. Worries about patents and competition suddenly tanked the stock to $60 within’ just 18 months – the stock had a massive swing in sentiment.
Did the once-celebrated star of the biotech world start hemorrhaging money? Was its revenue in a downward spiral? Far from it… In reality, 2018’s revenue saw only a minor dip from its peak in 2017, all the while the company was generating billions in profit.
Celgene gave it their all, teaming up with respected biotech experts, smart acquisitions of smaller companies with great prospective pipelines – even tried to engage Wall Street Critics, but nothing worked.
This twist in fortune caught the eye of Bristol Myers Squibb (NASDAQ:BMY), a company that values stability over trends. The lowered value of Celgene became a golden opportunity for them. They swooped in, acquiring Celgene, and securing promising assets. This stock market tale is a reminder that timing and how people perceive a situation can flip stock fortunes.
2. Great Science, but Lacking Funding:
Ever thought that a groundbreaking medicine should automatically become a hit? Turns out, it’s not that simple. Bringing a new medicine from idea to people’s medicine cabinets is like running an obstacle course. Yes, having solid science behind it is crucial, but that’s just the beginning. The American healthcare system has its quirks. Insurers need to give a nod, hospitals need to adapt to new methods, and doctors need to be convinced.
This process costs a lot of money—hundreds of millions, even a billion dollars—after all the scientific tests are done.
When you come across a small company that’s shown promising results in phase 2 or even phase 3 trials for a new drug, it’s time to dig into the numbers and dive into their financials. Check their balance sheet and cash flow. If financial jargon isn’t your strong suit, focus on a crucial question: “How fast are they spending their cash each quarter?” For instance, if they’re burning around $25 million per quarter and they only have $75 million left in the bank, alarm bells should ring.
Despite good results, a lack of funds could lead their breakthrough drug to end up in the trash bin. Smart financial moves are essential for turning promising data into real-world medical solutions.
This is where M&A steps in. Big pharma companies, with their knack for navigating red tape, join forces with agile biotech firms. M&A acts like a connector, ensuring innovative medicines actually reach the people who need them.
3. Key Shareholder: End of Career or Fresh Start?”
In the world of biotech, M&A isn’t just business—it’s about people and their journeys. Some founders, after years of battling the unpredictable industry, choose acquisition to secure their life’s work. On the flip side, young, ambitious founders, full of ideas, might be tempted by big corporations promising vast resources.
It’s not uncommon for a biotech company with a promising phase two product and solid data to command a valuation as high as $500 million. At this stage, the founding team has likely toiled in relative obscurity for years and looked to investors to fund their ambitious biotech endeavors.
Retaining majority control as a founder throughout product development is rare due to exorbitant costs. But the founder, or founding group, could retain around 5 to 10% of the company’s shares, possibly making them the largest and most influential shareholders. Hence, if Pfizer proposes to acquire the company for $500 million, that’s $50 million for the 67 year old scientist founder, who might just be tempted to take the giant payout and move to Bora Bora.
Another scenario involves young, inexperienced scientist-founders. This may seem counterintuitive; shouldn’t youth embody boldness?
Sometimes, these founders insist on going all the way. Yet, their inexperience can make them vulnerable to sophisticated corporate pitches. They’re promised abundant resources, a worry-free life, and a chance to change the world by teaming up with a giant like Pfizer.
Despite the allure, merging with massive corporations often stifles once-independent entrepreneurs. Ask anyone who’s sold their company to a corporate behemoth – it often isn’t conducive to the flourishing of once-autonomous entrepreneur.
Conclusion:
These tips can be crucial in spotting potential biotech takeovers, but there are still other factors to consider when piecing everything together. Even then, there’s no guarantee of a surefire deal. But if you make smart, educated guesses with thorough research, you can reap substantial returns – a little luck doesn’t hurt either.
Subscribe to Microcapdaily.com Right Now by entering your Email in the box below.
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Disclosure: We have not been compensated for this article/video. MicroCap Daily is not an investment advisor; this article/video does not provide investment advice. Always do your research, make your own investment decisions, or consult with your nearest financial advisor. This article/video is not a solicitation or recommendation to buy, sell, or hold securities. This article/video is our opinion, is meant for informational and educational purposes only, and does not provide investment advice. Past performance is not indicative of future performance.
Picture by Aymanejed from Pixabay
Recent Posts

Clean Vision Corp (OTC: CLNV): Overcoming the Plastic Waste Crisis

Meta Materials (NASDAQ: MMAT): More Due Diligence and Exploring Latest Developments

Integrated Cannabis Solutions’ (OTC: IGPK) 633% Surge: Exploring Catalysts, Company Overview, and Growth Potential in 2024

Sonoma Pharmaceuticals (NASDAQ: SNOA): Potential Surge to Speculations – What Lies Ahead?

1847 Holdings (NYSE: EFSH) Soars: Insights, Acquisitions, and What Lies Ahead
Trending
-

 Uncategorized2 years ago
Uncategorized2 years agoMeta Materials Inc (OTCMKTS: MMTLP) Enormous Short Position in Trouble as Next Bridge Hydrocarbons Set to Stop Trading (George Palikaras & John Brda on Corporate Action)
-

 Micro Cap Insider3 years ago
Micro Cap Insider3 years agoMedium (KOK PLAY) The Parabolic Rise of Metal Arts (OTCMKTS: MTRT)
-

 Media & Technology3 years ago
Media & Technology3 years agoHealthier Choices Management Corp. (OTCMKTS: HCMC) Powerful Comeback Brewing as PMI Patent Infringement Lawsuit Moves Forward
-
Media & Technology4 years ago
AMECA Mining RM; the Rise of Southcorp Capital, Inc. (OTCMKTS: STHC)
-

 Media & Technology3 years ago
Media & Technology3 years agoSNPW (Sun Pacific Holding Corp) Power Brewing: 50MW solar farm project in Durango Mexico MOU with Atlas Medrecycler 48,000 SF New Partnership Queensland Australia Solar Farm.
-

 BioPharma2 years ago
BioPharma2 years agoAsia Broadband (OTCMKTS: AABB) On the Move Northbound Since Sub $0.08 Dip as Crypto Innovator Elevates AABB Crypto Exchange & Enters the NFT Space
-

 Uncategorized2 years ago
Uncategorized2 years agoMeta Materials Inc (OTCMKTS: MMTLP) Short Squeeze S-1a4 Filing Signals S1 Approval Could Be Days Away (Next Bridge Hydrocarbons Spin-Off)
-
 BioPharma3 years ago
BioPharma3 years agoHumbl Inc (OTCMKTS: HMBL) Major Reversal as Powerful Advisor Rejoins the Team & Looks to Uplist to Major Exchange