Micro Cap Insider
Enzolytics, Inc. (ENZC) Major Breakout as Biotech Gains Traction in Booming HIV Market
Published
3 years agoon
By
Boe Rimes
Enzolytics, Inc. (ENZC) has emerged in recent weeks as the most exciting stock in small caps that is quickly developing a massive following of investors who are looking to ride this one past its recent 0.958 per share highs well into multi dollar land. ENZC is just now getting noticed by big money investors since the historic merger between BioClonetics and Enzolytics; the new biotech produces fully human monoclonal antibodies currently being employed to produce anti-SARS-CoV-2 (CoronaVirus) monoclonal antibodies for treating COVID-19 but more historically to fight HIV.
ENZC flagship is called ITV-1, this patented ITV-1 (Immune Therapeutic Vaccine-1) is a suspension of Inactivated Pepsin Fraction (IPF), which studies have shown is effective in boosting the imune system and controlling HIV/AIDS. ENZC just announced plans to take its ITV-1 anti-HIV therapeutic to clinical trials and distribution throughout Europe. The Company’s ITV-1 anti-HIV therapeutic earlier progressed toward certification under the Bulgarian Drug Agency (BDA) but that process was interrupted before completion. However, in that process, significant positive clinical trial results in patients were documented. These positive results give the Company total confidence that the now planned clinical trials under the European Medicines Agency (EMA) will likewise be successful. ENZC could become a major player in the enormous $30 billion annual HIV market expected to reach $37 billion in the next 5 years. In a market primarily controlled by Gilead who sold $17 billion in HIV drugs last year Enzolytic’s HIV treatment is immunotherapy, not chemotherapy as Insider Financial recently reported in an outstanding article on ENZC. ITV-1 appears to work across a range of infectious diseases, from coronaviruses to HIV by boosting the immune system and controlling HIV/AIDS most likely acting as a superantigen—inducing polyclonal expansion of T cells and stimulating a broad immune response against the virus, and by binding directly to gp41 and gp120 of the HIV virus.
Enzolytics Inc (OTCMKTS: ENZC) is a drug development Company committed to the commercialization of its proprietary proteins and monoclonal antibodies for the treatment of debilitating infectious diseases. The Company is advancing multiple therapeutics targeting numerous infectious diseases. One patented and clinically tested compound, ITV-1 (Immune Therapeutic Vaccine-1), is a suspension of Inactivated Pepsin Fraction (IPF), which studies have shown is effective in treating HIV/AIDS. IPF is the active component of ITV-1 and is a purified extract of porcine pepsin. ITV-1 has also been shown to modulate the immune system. The Company is also implementing its proprietary technology to produce fully human monoclonal antibodies (mAbs) against infectious diseases, including HIV, rabies, influenza A, influenza B, tetanus, and diphtheria. Its proprietary methodology for producing fully human monoclonal antibodies is currently employed to produce monoclonal antibody therapeutics for numerous infectious diseases, including the Coronavirus (SARS-CoV-2) and HTLV-1.
ENZC has partnered with Intel to publish a white paper titled, “Optimizing Empathetic A.I. to Cure Deadly Diseases,” highlighting Intel’s Artificial Intelligence Analytic tools and Enzolytic’s innovative approach and groundbreaking contributions to create universal, durable, and broadly effective treatment targeting all virus variants. This collaborative effort approaches the future of medicine; a future wherein the process of healthcare evolves from reactive to anticipatory, as exemplified by P4 Medicine. The conclusion described in the White Paper is that Enzolytics Inc’s use of Artificial Intelligence underscores a novel approach in assessing millions of virus sequences to identify the conserved segments essential for virus survival. Through effective public and private sector symbiosis and the fostering of a technology-neutral regulatory environment, the genuine power of A.I., coupled with advanced techniques in proprietary technologies illustrated in the Enzolytics immunotherapeutics, will no doubt create universal, durable, and broadly effective treatment targeting all virus variants. This was followed by the news that ENZC has identified conserved, expectedly immutable sites on the HTLV-1 virus against which it will produce targeted anti-HTLV-1 monoclonal antibodies (mAbs). Utilizing the Company’s proprietary Artificial Intelligence (AI) methodology, conserved target sites have now been identified against which fully human anti-HTLV-1 monoclonal antibodies will be produced in its lab on the campus of Texas A&M University in the University’s Institute for Preclinical Studies. The Company’s own Artificial Intelligence team continues to identify the critical sites on viruses against which monoclonal antibodies will be produced to treat infectious viral diseases.
Microcapdaily has been reporting on the ENZC BioColnetics merger since the beginning recenlty stating: “Enzolytics Inc. ENZC: is making a highly explosive move up the charts recently surpassing $0.50 per share and regularly topping $25 million USD per day in dollar volume ENZC has transformed into a major league runner in small caps. Enzolytics and its new subsidiary BioClonetics own licensing rights of the Irreversible Pepsin Fraction peptide molecule for the treatment of HIV/AIDS a market expected to be worth $30 billion plus by 2025. The Company produces targeted (nontoxic) monoclonal antibodies and is now advancing two separate but complementary therapy platforms for treating infectious diseases, targeting HIV and the CoronaVirus. Enzolytics has attracted a major league level management team behind it and has expanded its lab capabilities on the Texas A&M University campus at the Institute for Pre-clinical Studies, where it is producing both addition monoclonal antibodies against HIV and the against covid-19. This expansion allows Enzolytics to complete the production of monoclonal antibodies against both the HIV virus and the coronavirus and collaborate with the biopharma experts on the campus. Microcapdaily first reported on ENZC the day after the merger was announced in our article: “BioClonetics LOI Sparks Enzolytics Inc (OTCMKTS: ENZC)” on September 16 when ENZC was trading for well under $0.01
Enzolytics has quickly attracted a power house team behind it which speaks of big things to come here. They recently appointed Ronald Moss, M.D., to the Medical Advisory Board. Mr. Moss has been an executive with numerous biotech’s over the past 25 years. He has extensive clinical and regulatory management expertise in guiding programs through Phase I, II, and III clinical trials, including IND and NDA experience. The Company’s Chief Science Officer, Mr. Henry Zhabilov has managed several clinical trials utilizing therapeutic proteins. He is the inventor of several U.S. patents related to the immunotherapy of HIV and cancer and an immune enhancer based on the company’s IPF platform.
$ENZC : Bingo ! https://t.co/bKc0TKwLvp
— Super-Trend Master (@Bluew3312) June 30, 2021
$ENZC Big week ahead. Perfect loading zone to be a part of the takeover! It's time for the #CURE @enzolytics https://t.co/cJxCsA06Ju
— A10-Vet (@A10_Vet) July 2, 2021
To Find out the inside Scoop on ENZC Subscribe to Microcapdaily.com Right Now by entering your Email in the box below
On June 14 ENZC announced it has concluded definitive plans to advance its previously tested ITV-1 anti-HIV therapeutic to clinical trials and distribution throughout Europe. Completion of these steps will establish its anti-HIV therapy as a significant revenue source for the Company, a meaningful milestone for both human health and Company profitability. The Company’s ITV-1 anti-HIV therapeutic earlier progressed toward certification under the Bulgarian Drug Agency (BDA) but that process was interrupted before completion. However, in that process, significant positive clinical trial results in patients were documented. These positive results give the Company total confidence that the now planned clinical trials under the European Medicines Agency (EMA) will likewise be successful.
The steps now in place to accomplish the Company’s goal of bringing its anti-HIV ITV-1 therapeutic to patients are: The Company has formed International Medical Partners Ltd. (IMPL) in partnership with European partners. IMPL is owned equally by the Company and its partners and has the licenses to distribute the ITV-1 therapeutic in the 27 European countries covered by the European Medical Agency plus Russia, Georgia, Ukraine, Moldova, Belarus, Armenia, Azerbaijan, Kazakhstan, Uzbekistan, Turkmenistan, Kyrgyzstan, Tajikistan, Estonia, Latvia and Lithuania.
The Company has engaged the Contract Manufacturing Company (CMO) Danhson Ltd. to produce the initial quantity of ITV-1 used in preparing the required Best Methods Report for future production and clinical trials documentation.The Company has engaged the Contract Research Organization (CRO) Clinical Design Ltd. in cooperation with PharmaLex to prepare and establish a drug development programme concerning creation of protocols for human clinical trials that will lead to the licensing of the Company’s ITV-1 therapeutic under the European Medicine Agency (EMA). The production at Danhson Ltd. will produce initial quantities of the therapeutic to be used for completion of preclinical testing and then initiating clinical trials with Clinical Designs, Ltd. MBL is contracting with PharmaLex, a leading EU regulatory consulting company to manage document review of the product compliance with EMA requirements.
CEO Charles Cotropia said, “The Company’s objective is to provide new, better and safer therapeutics to treat HIV. Currently, treatment is solely through the lifelong use of antiretrovirals (ARVs) which are expensive and have long lasting, serious negative effects on the body. The costs for such ARVs are excessive – from $27,540 per year for Dovato by ViiV/Glaxo SmithKline to $42,635 per year for Biktarvy by Gilead and a lifetime costs of up to $350,000 – and even then, only 54% of those ineffective by HIV have access to such treatments – leaving 46% with no treatment. New and improved therapies, that are less expensive and more effective, are desperately needed. Providing these better therapies are our Company’s objective.”
$ENZC: YES !!! https://t.co/ujTNhxkM9u
— Elvis747man (@elvis747man) July 1, 2021
https://twitter.com/Dr_Stonky/status/1409545986918305801
For more on ENZC Subscribe Right Now!
Enzolytics has emerged in recent weeks as the most exciting stock in small caps that is quickly developing a massive following of investors who are looking to ride this one past its recent 0.958 per share highs well into multi dollar land. ENZC is just now getting noticed by big money investors since the historic merger between BioClonetics and Enzolytics; the new biotech produces fully human monoclonal antibodies currently being employed to produce anti-SARS-CoV-2 (CoronaVirus) monoclonal antibodies for treating COVID-19 but more historically to fight HIV. ENZC flagship is called ITV-1, this patented ITV-1 (Immune Therapeutic Vaccine-1) is a suspension of Inactivated Pepsin Fraction (IPF), which studies have shown is effective in boosting the imune system and controlling HIV/AIDS. ENZC just announced plans to take its ITV-1 anti-HIV therapeutic to clinical trials and distribution throughout Europe. The Company’s ITV-1 anti-HIV therapeutic earlier progressed toward certification under the Bulgarian Drug Agency (BDA) but that process was interrupted before completion. However, in that process, significant positive clinical trial results in patients were documented. These positive results give the Company total confidence that the now planned clinical trials under the European Medicines Agency (EMA) will likewise be successful. ENZC could become a major player in the enormous $30 billion annual HIV market expected to reach $37 billion in the next 5 years. In a market primarily controlled by Gilead who sold $17 billion in HIV drugs last year Enzolytic’s HIV treatment is immunotherapy, not chemotherapy as Insider Financial recently reported in an outstanding article on ENZC. ITV-1 appears to work across a range of infectious diseases, from coronaviruses to HIV by boosting the immune system and controlling HIV/AIDS most likely acting as a superantigen—inducing polyclonal expansion of T cells and stimulating a broad immune response against the virus, and by binding directly to gp41 and gp120 of the HIV virus. Microcapdaily first reported on ENZC on September 16 when the stock was well below a penny. We will be updating on ENZC when more details emerge so make sure you are subscribed to Microcapdaily so you know what’s going on with ENZC.
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Disclosure: we hold no position in ENZC either long or short and we have not been compensated for this article.
You may like
-


Enzolytics Inc (OTCMKTS: ENZC) Biotech Reversal Underway As ITV-1 Gains Traction
-


Enzolytics Inc (OTCMKTS: ENZC) Coiled Tight Near 52 Week Lows as Biotech Looks to Disrupt Several Enormous Markets (ITV-1 for HIV, Monoclonal Antibodies & AI)
-


Enzolytics Inc (OTCMKTS: ENZC) Powerful Reversal Underway as Immune™ Goes International in Booming Nutritional Supplement Market
-


Enzolytics Inc (OTCMKTS: ENZC) Running Up the Charts After Biotech Outlines Progress and Future Plans (Update on: Artificial Intelligence Diagnostics & ITV-1 and IPF Immune)
-


Enzolytics Inc (OTCMKTS: ENZC) Major Reversal Brewing as Biotech Gains Traction in HIV Market (Update on: ITV-1 EMA Approval, ITV-1, anti-HIV Treatment & IPF Immune US Launch)
-


Enzolytics, Inc. (ENZC) Big Reversal Northbound as Biotech Launches IPF Immune & Plans Immune Therapeutic ITV-1 for Clinical Trials
3 Comments
Leave a Reply
Cancel reply
Leave a Reply
Featured
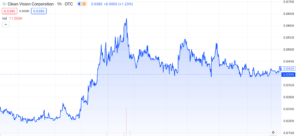
Clean Vision Corp (OTC: CLNV): Overcoming the Plastic Waste Crisis
Published
6 months agoon
January 18, 2024
Clean Vision Corporation (OTC: CLNV) has experienced several interesting developments recently, but it hasn’t noticeably influenced the market with any substantial gains. Nonetheless, we believe it’s worth providing an update on the company given it’s been a few months since our last mention. In today’s discussion, we’ll explore a variety of updates and their significance, with aim of providing insight on what to expect for 2024.
Background:
Clean Vision is led by Dan Bates, and their goal is to tackle the global plastic waste crisis head-on. Their wholly owned subsidiary, Clean Seas, has developed the Plastic Conversion Network (PCN), a groundbreaking technology aimed at diverting millions of tons of waste plastic from landfills, incineration, and oceans. The PCN converts this plastic feedstock into clean fuels and green hydrogen, significantly reducing reliance on fossil fuels and lowering the carbon footprint.
For a brief 2 minute overview on the company, feel free to reference the video CLNV’s subsidiary put together on YouTube. Here’s the link.
Clean Seas utilizes proven pyrolysis technology to produce environmentally friendly products, which are sold to multinational petrochemical companies, driving the circular plastic economy. Operational PCN facilities are already in place in Morocco and India, with additional conversion facilities in development across West Virginia, Arizona, and Southeast Asia. Long-term feedstock supply agreements exceeding one million tons of waste plastic annually have been secured at no cost.
Their recently trademarked brand, AquaH®, is produced in their PCN. According to the release, it offers a differentiated green hydrogen product from carbon-neutral sources. Currently, hydrogen is predominantly produced through methods that involve fossil fuels, which of course contributes to global carbon emissions. Furthermore according to Deloitte’s 2023 global green hydrogen outlook, this could be a $1.4T annual market by 2050.
$65 Million Plastic Conversion Facility:
CLNV is making big moves in West Virginia and according to the release on October 24th, 2023, they’ve brought in some serious players—CDI Engineering Solutions and ERM—to help out with their Clean-Seas West Virginia project.
CDI has over 70 years of experience integrating engineering, design, project support, procurement and construction management services to the energy, chemicals and electrical infrastructure markets.
ERM is the world’s largest advisory firm focused solely on sustainability, offering environmental, health, safety, risk and social expertise for more than 50 years with more than 8,500 dedicated professionals operating across 40 countries.
The plan is to kick things off in 2024, turning 100 tons of plastic every day into recycled plastics and clean fuels. It’s a hefty project with a $65 million investment, creating over 200 jobs initially. And they’re not stopping there—they want to scale up to 500 tons of plastic per day over time.
West Virginia Governor Jim Justice is also on board, throwing over $12 million in state incentives to support the project.
Governor Jim Justice made a reference to Clean Seas in his state of the union address. If you want to catch the mention, go to 34:15 in the video. The three minutes leading up to it are also worth reviewing.
Subscribe to Microcapdaily.com Right Now by entering your Email in the box below.
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Launches Global Operations:
CLNV made another significant advancement, planning to launch waste plastic conversion facilities in the European Union, Eastern Europe, and Southeast Asia. This will be accomplished through their new subsidiary, Clean-Seas Partners UK Limited (CS-UK), who of course shares the same vision of creating sustainable solutions to the global plastic pollution crisis.
Under the leadership of Managing Director Shaun Wootton, CS-UK will play a crucial role in strategic project development and investment facilitation, leveraging established relationships in the Middle East, Southeast Asia, and Europe.
To fortify effective governance and strategic direction, CS-UK is assembling a distinguished board with internationally recognized figures in banking, sustainability, and energy. This approach aims to have a diverse and experienced board guiding CS-UK in realizing its vision of promoting sustainability and environmental stewardship across diverse regions.
$340 Million Bond Offering:
CLNV even announced they partnered with a global advisory firm, Grant Thornton, to issue up to $340 million in Green Bonds. This is the world’s sixth-largest network of independent accounting and consulting firms, employing 62,000 people in more than 130 countries and had revenues of $6.6 billion in 2021. These bonds will fund the expansion of Clean Vision’s Plastic Conversion Network (PCN) under the “Clean-Seas” initiative worldwide, aimed at combatting plastic pollution on a global scale.
With the Green Bond’s net proceeds, CLNV plans to deploy at least six plastic waste conversion lines globally, with strategic locations in West Virginia, Arizona, Southeast Asia, and expansion in Morocco. The Green Bond is also expected to attract environmentally conscious investors, setting a new standard for corporate responsibility.
$15M Government Loan:
Lastly, under the capable management of Huntington Bank, CLNV has recently secured a $15 million government loan. What sets this apart is that the loan is FORGIVABLE.
A forgivable loan is a type of loan where the borrower is not required to repay the borrowed amount under certain conditions. Typically, these conditions are related to the borrower meeting specific criteria, such as using the funds for approved purposes, maintaining certain employment levels, or achieving predetermined goals. If the borrower fulfills these conditions, the loan is forgiven, and they are not obligated to repay the borrowed amount. Forgivable loans are often used as an incentive or support for specific activities, such as job creation, small business development, or other initiatives that contribute to economic growth or community welfare.
Not to mention it won’t result in any dilution for shareholders. This is an unexpected and uncommon accomplishment for an OTC company. Securing a government loan of this size without any dilution is truly impressive.
Conclusion:
CLNV has made impressive strides tackling the global plastic waste crisis, especially given their valuation of merely $22.65 million. The team has swiftly achieved key objectives, including a $65 million plastic conversion facility in West Virginia, global expansion through Clean-Seas Partners UK Limited, a $340 million Green Bond Offering, and a remarkable $15 million forgivable government loan. The vast $1.4 trillion market they’re tapping into offers an interesting opportunity with current indicators looking positive. Nevertheless, it’s crucial to acknowledge that there is still significant work ahead, and the team needs to maintain consistent execution to turn this potential into a reality.
We will update you on CLNV when more details emerge, subscribe to Microcapdaily to follow along!
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Disclosure: We have not been compensated for this article/video. MicroCap Daily is not an investment advisor; this article/video does not provide investment advice. Always do your research, make your own investment decisions, or consult with your nearest financial advisor. This article/video is not a solicitation or recommendation to buy, sell, or hold securities. This article/video is our opinion, is meant for informational and educational purposes only, and does not provide investment advice. Past performance is not indicative of future performance.
Picture by pasja1000 from Pixabay
Featured
Meta Materials (NASDAQ: MMAT): More Due Diligence and Exploring Latest Developments
Published
6 months agoon
January 16, 2024
Meta Materials (NASDAQ: MMAT) witnessed a significant uptick in trading activity on January 16th, 2024, resulting in a notable 20% increase in its stock value by market close. Intrigued by this surge, we explored various sources, including press releases, SEC filings, and social media, to identify the catalyst behind this sudden gain.
Unexpectedly our research revealed no recent material releases. Instead, the surge seems tied to an announcement from a few days ago that didn’t grab much attention at first. As time passed, it started generating more buzz but there’s still a lot more to dig into and a number of ideas to consider for today’s rally.
If you haven’t caught up on our previous analyses of MMAT, you can find the overview here. In this report, we aim to explore the cause-and-effect dynamics of recent events, offering insights that might illuminate expectations for Meta Materials in the near future.
Background:
If you’re new to MMAT or haven’t been a long-time follower, let’s kick things off with a quick intro to the company.
Meta Materials stands at the forefront of advanced materials and nanotechnology. Their focus is on pioneering novel products and technologies utilizing sustainable and innovative scientific approaches. The interesting part is their advanced materials have the transformative power to enhance a variety of common products, infusing them with heightened intelligence and sustainability.
Leveraging its technology platforms, they’re capable of empowering global brands in creating cutting-edge products that elevate overall performance.
Their technology has application across multiple industries including aerospace and defense, consumer electronics, 5G communications, batteries, authentication, automotive, and clean energy. Their agreement with Panasonic is certainly a great start to empowering their growth in one of many verticals. Overall the TAM is ~$32B and with current growth rates, it’ll increase to a whopping ~$61B by 2026.
MMAT’s goal is to shape a smarter and more sustainable world. If you look through their presentation, you can continue to evaluate the many ways their technology transforms everyday lives. We highly suggest you take a look.
Additional Resources:
- @LauraLoomer’s video on MMAT
- @metaheadj’s post on X, displaying Rob Stone‘s response update for an investor
Subscribe to Microcapdaily.com Right Now by entering your Email in the box below.
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
What Happened:
So, MMAT issued a press release on January 11th, 2024, announcing a proposed settlement with the Securities and Exchange Commission (SEC) concerning an investigation related to the Torchlight Energy Resources, Inc. and Metamaterial Inc. merger.
According to the release, The company has extended a settlement offer (Proposed SEC Settlement) to the SEC’s Division of Enforcement. This proposed settlement aims to address concerns regarding antifraud, reporting, books and records, and internal accounting control provisions of securities laws. It is important to note that the Proposed SEC Settlement is contingent on approval by the SEC Commissioners, and the company cannot predict the approval timeline.
If accepted, the Proposed SEC Settlement would involve the SEC entering a cease-and-desist order and the company paying a civil money penalty of $1 million over a one-year period in four installments. Notably, the company would neither admit nor deny the findings outlined in the Order.
The company’s board of directors and management team view the Proposed SEC Settlement as beneficial for shareholders. If approved, it is expected to remove uncertainty surrounding the investigation, enabling the company to focus on advancing its business objectives.
So What:
If you’ve just read through the announcement and are confused, you’re not alone. It appears that many investors may have mis-read the press release, thinking that the SEC was being punished and MMAT was reaching a settlement agreement, but it appears to be the other way around.
In the event of approval, the company is obligated to pay a civil money penalty of $1 million. This penalty would be paid in four installments over the course of one year, following an agreed-upon payment plan. However, the PR also notes that the company cannot predict with certainty whether or when the Proposed SEC Settlement will even be approved by the SEC Commissioners.
According to another user on X, @AShortSqueeze, MMAT’s initial analysis has potentially revealed the motherload of counterfeit shares.
But if you scroll through the comments, you’ll see other users pointing out that this information is actually old news. This is just one of many widely circulated posts that might have been misunderstood.
Significant Coverage:
Another theory suggests that a notable influencer in the financial space, @MoonMarket_, has set their sights on the company and is conducting additional due diligence. With a substantial following of almost 75K users, the influencer’s involvement could have contributed to a significant fluctuation in today’s trading session. It’s important to recognize that X is packed with plenty of financial influencers, and blindly following their moves can be risky. Many are involved in day trades, momentum trading, or at least contemplating such strategies.
Conclusion:
The buzz around MMAT today seems fuelled by a mix of misrepresented themes and recycled news, creating the illusion of fresh, imminent developments.
As per usual, the magnitude of MMAT’s technology and potential integrations across various verticals continues to create a roar of excitement. On another front, we’re also continuing to see speculation about a short squeeze due to substantial amounts of counterfeit shares.
For now, patience is key and we suggest closely monitoring developments. MMAT especially tends to be quite volatile.
Disclosure: We have not been compensated for this article/video. MicroCap Daily is not an investment advisor; this article/video does not provide investment advice. Always do your research, make your own investment decisions, or consult with your nearest financial advisor. This article/video is not a solicitation or recommendation to buy, sell, or hold securities. This article/video is our opinion, is meant for informational and educational purposes only, and does not provide investment advice. Past performance is not indicative of future performance.
Picture by StartupStockPhotos from Pixabay
Featured
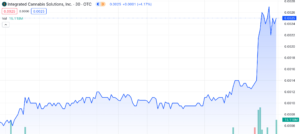
Integrated Cannabis Solutions’ (OTC: IGPK) 633% Surge: Exploring Catalysts, Company Overview, and Growth Potential in 2024
Published
7 months agoon
January 12, 2024
Integrated Cannabis Solutions (OTC: IGPK) has undergone a remarkable uptrend, surging an impressive 633% since December 11th, 2023, with 166% of that surge taking place across yesterday’s trading session and today, January 11th, 2023. Both days have been marked by unprecedented volume – Yahoo Finance reported an almost 30x increase, with 115,867,027 shares traded by close yesterday. We’re already seeing 90,092,317 shares traded this morning and it’s just barely noon. Today we’ll explore the catalysts behind the surge, offer a comprehensive overview of the company, and evaluate IGPK’s potential for sustained growth throughout 2024.
Background:
Let’s get straight to it. IGPK is the result of a recent reverse merger with Integrated Cannabis Solutions and JFH Digital E-Commerce Corp. The first thing you’ll notice is finding the website isn’t a walk in the park, we’re fairly certain there isn’t one yet, at least one that will help in any way related to more investment information. Your best bet for more any information is to check out IGPK’s OTC Market page for details, but even the company description on there is not accurate. We’ve mainly found the following information through filings, IGPK’s Twitter, and other online users.
Keep in mind this breakdown might not be flawless given we’re piecing it together mostly from what folks on X are saying. But we’ll try our absolute best to lay it all out for you.
IGPK appears to have been a shell for little while until JFH stepped in. A user on X, @stockplayer30, broke it down fairly simply, stating that the shell’s slate was wiped clean, cancelling all notes payable and any debt. Whether it’s a promissory note, convertible note, or convertible debenture, the main point is they ditched all debt. JFH has an opportunity to start fresh, and it certainly makes this deal a lot more interesting.
Just a heads up, it’s a Chinese merger. If the idea of a Chinese leadership team makes you a bit wary, you might want to pause here. However if you were in the trading game during the summer of ’23, you probably remember those crazy spikes in some Chinese Nasdaq deals. And get this – no big press releases or SEC filings to explain those sudden jumps either.
Subscribe to Microcapdaily.com Right Now by entering your Email in the box below.
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Company Description:
Onto what the company actually does. According to @SuperRobotOTC on X, this is a digital e-commerce company based in China, here’s a link to their e-commerce website. This user also put together a great overview of the company on YouTube, if you’d like to watch something informative in video format, click here.
Another user, @igal_n, found a blurb on the company that states, “Junfenghuang (JFH) is a digital asset. It is a token issued by Uplus Future Company with the help of blockchain technology. It has no direct relationship with the original equity”.
The Potential:
What makes this story extremely interesting is the sheer magnitude of how large JFH is, the intrinsic value does not appear to be valued accurately in the market, given it’s only freshly merged into IGPK’s tiny shell company on the OTC.
Their Gross Merchandise Value (GMV) is heading north of 50 billion yuan, and post-merger profits from service outlets are looking at a hefty 10 billion yuan – yes, billion with a B.
Steering the ship is a leadership team featuring President Wang Dejun, Treasurer Xie Weiji, and Director Yang Lanfang. With a whopping 750 subsidiaries, 250,000 merchants, and 30 million registered users. We’ve also heard from other sources that the registered users could be nearly double that, coming in at 50 million registered users.
These numbers are substantial for a company with a $7 million market cap. Looking ahead, it won’t be shocking if IGPK sets its sights on moving up to a bigger exchange like NASDAQ. It’s no secret they’re already in the big leagues – or it at least appears so. If that were the case, they’d of course have enhanced credibility, more visibility, and increased access to capital with institutional funding.
The App:
Now, you might be wondering, “Sounds cool, but it’s a Chinese merger with a whole setup on the other side of the planet. Can we trust this info?” @SuperRobotOTC has also gone the extra mile by downloading the app, and gave us the lowdown in video format. On top of the SEC filings, this is an added layer of trust & credibility we can attribute to this new venture. Here’s the link to the video.
Conclusion:
Fortunately it appears IGPK is still for the most part flying under the radar. There’s not even a proper website or accurate update on IGPK’s OTC Market overview to tell us what the company even entails. But here’s the silver lining – that might mean you’re still early. The intrinsic value of IGPK appears strongly disproportionate to its current value in the market.
Our advice? Keep a close eye on IGPK’s journey as it takes on this exciting phase of growth and exploration. It’s likely this story will catch wind quickly and it could be a great time to take advantage. As @SuperRobotOTC eluded to in his video, this could be the OTC’s largest merger, with a potential $70B valuation.
We will update you on IGPK when more details emerge, subscribe to Microcapdaily to follow along!
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Disclosure: We have not been compensated for this article/video. MicroCap Daily is not an investment advisor; this article/video does not provide investment advice. Always do your research, make your own investment decisions, or consult with your nearest financial advisor. This article/video is not a solicitation or recommendation to buy, sell, or hold securities. This article/video is our opinion, is meant for informational and educational purposes only, and does not provide investment advice. Past performance is not indicative of future performance.
Picture by geralt from Pixabay
Recent Posts

Clean Vision Corp (OTC: CLNV): Overcoming the Plastic Waste Crisis

Meta Materials (NASDAQ: MMAT): More Due Diligence and Exploring Latest Developments

Integrated Cannabis Solutions’ (OTC: IGPK) 633% Surge: Exploring Catalysts, Company Overview, and Growth Potential in 2024

Sonoma Pharmaceuticals (NASDAQ: SNOA): Potential Surge to Speculations – What Lies Ahead?

1847 Holdings (NYSE: EFSH) Soars: Insights, Acquisitions, and What Lies Ahead
Trending
-

 Uncategorized2 years ago
Uncategorized2 years agoMeta Materials Inc (OTCMKTS: MMTLP) Enormous Short Position in Trouble as Next Bridge Hydrocarbons Set to Stop Trading (George Palikaras & John Brda on Corporate Action)
-

 Micro Cap Insider3 years ago
Micro Cap Insider3 years agoMedium (KOK PLAY) The Parabolic Rise of Metal Arts (OTCMKTS: MTRT)
-

 Media & Technology3 years ago
Media & Technology3 years agoHealthier Choices Management Corp. (OTCMKTS: HCMC) Powerful Comeback Brewing as PMI Patent Infringement Lawsuit Moves Forward
-
Media & Technology4 years ago
AMECA Mining RM; the Rise of Southcorp Capital, Inc. (OTCMKTS: STHC)
-

 Media & Technology3 years ago
Media & Technology3 years agoSNPW (Sun Pacific Holding Corp) Power Brewing: 50MW solar farm project in Durango Mexico MOU with Atlas Medrecycler 48,000 SF New Partnership Queensland Australia Solar Farm.
-

 BioPharma2 years ago
BioPharma2 years agoAsia Broadband (OTCMKTS: AABB) On the Move Northbound Since Sub $0.08 Dip as Crypto Innovator Elevates AABB Crypto Exchange & Enters the NFT Space
-

 Uncategorized2 years ago
Uncategorized2 years agoMeta Materials Inc (OTCMKTS: MMTLP) Short Squeeze S-1a4 Filing Signals S1 Approval Could Be Days Away (Next Bridge Hydrocarbons Spin-Off)
-
 BioPharma3 years ago
BioPharma3 years agoHumbl Inc (OTCMKTS: HMBL) Major Reversal as Powerful Advisor Rejoins the Team & Looks to Uplist to Major Exchange



Lois Dickson Myers
July 5, 2021 at 1:18 am
Nice Article, but it didn’t mention its other Premiere Patent: Clone3, which is so valuable Dr. Fauci tried to steal it in 1998, falsely claming he invented it, but US Patent Examiner ruled in favor of the true inventor, Dr. Joseph Cotropia, of BioClonetics, Dallas, TX, whose brother Charles Cotropia, a seeasoned patent lawyer, defended him at this Hearing, and won the Case. When BioClonetics merged with Enzolytics in Dec. 2020, Charles Cotropia was appointed CEO of the Company. Currently being tested to work synergistically with ITV-1, Clone3 is the only all-human-all-natural monoclonal antibody cure for infectious diseases, including COVID & HIV-AIDS.
Boe Rimes
July 5, 2021 at 11:15 am
THanks Lois very important point, we will make sure to include it in our next article on ENZC. Thank you!
Marvin
July 5, 2021 at 3:49 pm
Excellent! I was wondering if clone3 was going to be mentioned. Is clone3 seperate from ITV-1?